Anti-HLA Antibodies in Lung Transplantation
Department of Pathology, University of California at San Diego, 9500 Gilman Drive, MC 0612, La Jolla, CA 92093, USA
Received: February 20, 2017 | Accepted: April 6, 2017 | Published: April 11, 2017
OBM Transplantation 2017, Volume 1, Issue 1, doi:10.21926/obm.transplant.1701002
Academic Editor: Kamyar Afshar
Recommended citation: Morris GP. Anti-HLA Antibodies in Lung Transplantation. OBM Transplantation 2017; 1(1): 002; doi:10.21926/obm.transplant.1701002.
© 2017 by the author. This is an open access article distributed under the conditions of the Creative Commons by Attribution License, which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is correctly cited.
Abstract
(1) Background: Lung transplantation is an increasingly utilized treatment for end-stage lung disease. Scarcity of organ donors limiting transplantation underscores the importance of optimal histocompatibility testing approaches to facilitate organ allocation and avoid immunologic rejection. Significant data has emerged over the past decade to define the role of alloantibodies against HLA in the pathogenesis of post-lung transplant complications. (2) Methods: Medical literature from 1996–2016 related to search term “lung transplant” was reviewed using PubMed. (3) Results: Review of published literature demonstrated a progressive understanding of the contributions of anti-HLA antibodies to antibody-mediated rejection, bronchiolitis obliterans syndrome, and chronic lung allograft dysfunction. This coincided with implementation of refined histocompatibility testing methods with improved sensitivity and specificity. (4) Conclusions: Antibodies against donor-specific HLA (DSA) contribute to the pathogenesis of immune-mediated complications after lung transplantation. Detection of DSA in peripheral blood is associated with poor outcomes. Current histocompatibility methods have improved prognostic and diagnostic testing to assist directing patient care.
Keywords
lung transplant; HLA; antibody; DSA; histocompatibility
Introduction
Lung transplantation is an effective therapy for end-stage lung disease. Recognition of the allograft as foreign by the immune system can cause cellular and humoral immune responses leading to graft rejection. Immunostimulatory polymorphisms on HLA molecules are the main drivers of alloimmune responses. Limits to organ availability preclude HLA matching for a majority of transplants. Instead, histocompatibility testing focuses on avoiding pre-existing humoral immunity against donor alloantigens and immunosuppression to limit development of de novo alloimmune responses after transplant. The role of histocompatibility testing in lung transplantation has changed significantly in the past 15 years, moving from minimal consideration for pre-transplant testing toward current approaches of highly sensitive and specific testing aimed at avoiding transplantation against specific donor alloantigens to which the patient has antibodies and routine post-transplant monitoring for evidence of donor-specific antibodies (DSA) associated with allograft dysfunction. This review provides a historical perspective and examination of published evidence supporting histocompatibility testing in lung transplantation.
Results
Histocompatibility in Lung Transplantation
Advances in patient and donor medical management, immunosuppressive treatments, surgical techniques, and histocompatibility testing have combined to enable successful transplantation of over 27,000 patients in the United States each year (based on OPTN data as of December 30 2016) [1]. While kidney and liver transplants have continually constituted the majority of solid organ transplants in the US, lung transplants have increased dramatically over the past 20 years, from 815 in 1996 to 1405 in 2006 to 2122 in 2016. The success of lung transplantation, much like other transplanted organs, depends on several factors including immunologic compatibility between donor and recipient. However, the relatively recent expansion of lung transplantation has meant that there is uncertainty as to which factors most significantly influence outcomes. The long history of kidney transplantation provides the largest data set in transplantation, with clear evidence that histocompatibility and immune-mediated rejection are critical, if not the most significant, factors for both peri-transplant and long-term outcomes [2]. A straight-forward proof-of-concept for the benefit of pre-transplant histocompatibility testing is illustrated by antibodies against ABO blood groups, which are capable of mediating hyperacute rejection [3]. While antibodies against non-self ABO antigens are universal, there are only four major ABO blood types (A, B, AB, and O) which permit relatively easy donor selection based on ABO compatibility and avoidance of ABO antibody-mediated hyperacute rejection.
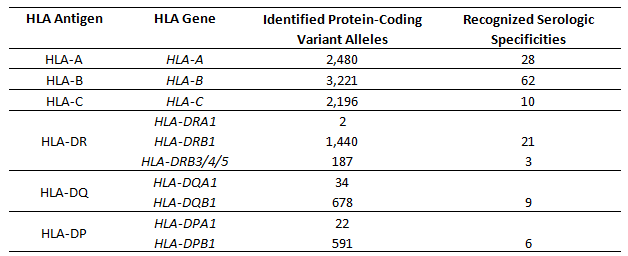
Lung transplantation is similarly susceptible to immune-mediated insult and rejection; current national data demonstrate that 17.2% of patients receiving a lung transplant in 2012–2013 experienced at least one acute rejection episode and that graft dysfunction driven by immune-associated pathology is the leading cause of post-transplant 5-year mortality [1]. Both the cellular and humoral arms of the immune system are capable of mediating lung allograft rejection. In both cases, the primary targets are Human Leukocyte Antigens (HLA) (Table 1). HLA molecules are the human orthologs of MHC molecules, which serve an essential role for presenting antigens to T lymphocytes. HLA genes are the most polymorphic in the human genome, with nearly 8,000 protein-coding variants for the genes encoding class I HLA-A, -B, and -C and over 3,000 such variants for the class II HLA-DR, -DQ, and -DP [4]. These allotypic variations are functionally relevant, as they influence the antigens presented to T cells and can be recognized as foreign by antibodies. It is estimated that each HLA gene product presents as many as 2x104 different endogenous antigens on the cell surface, and that the composition of the antigenic repertoire can vary completely between alleles of the same HLA gene, thus providing ample stimulation for rejection of allografts [5]. Given the extensive population diversity of HLA genes and the limited donor pool, it is not feasible to match patients to donors with identical HLA alleles. As it is not possible to avoid T cell-mediated alloreactive responses against lung allografts by histocompatibility and donor selection, prevention and treatment for cellular rejection relies on extensive and life-long immunosuppression [6,7].
Table 1 HLA genes.
A role for alloantibody-mediated pathology in lung transplantation has become increasingly recognized as a source of allograft dysfunction and post-transplant morbidity and mortality [8]. Antibodies recognizing HLA can mediate rejection through multiple pathogenic mechanisms including activation of complement and induction of cell lysis, recruitment of immune cells with cytolytic function, and inducing physiologic changes to target cells via signalling resulting from HLA ligation [9]. Antibody-mediated rejection (AMR) is a well-described phenomenon in kidney [2] and heart transplantation [10]. In lung transplantation, AMR can be classified based on timing, with hyperacute rejection occurring peri-operatively, acute rejection occuring as discrete clinically-symptomatic episodes, and chronic rejection manifesting as persistent allograft dysfunction resulting from cumulative and ongoing pathologic events. Current standards for diagnosis of AMR in lung transplantation include the presence of anti-HLA antibodies against donor HLA antigens as an equal criterion along with allograft dysfunction, biopsy histologic features consistent with AMR, detection of C4d deposition on biopsy, and exclusion of other sources of allograft dysfunction [8]. The presence of alloantibodies against donor HLA antigens is significantly correlated with histologic findings consistent with AMR, demonstrating a physiologic linkage [11]. Subclinical AMR is recognized as a distinct entity, with evidence of antibody-mediated pathologic processes in the absence of significant allograft dysfunction.
Alloantibodies also contribute to more chronic allograft dysfunction in bronchiolitis obliterans syndrome (BOS) [11] and chronic lung allograft dysfunction (CLAD) [13]. It is thought that the pathophysiologic processes of AMR, CLAD, and BOS may be related, with subclinical AMR preceding episodes of acute AMR which evolve into syndromes of chronic lung dysfunction such as chronic AMR, CLAD, or BOS. However, there is insufficient data to definitively demonstrate causal linkage between these phenomena.
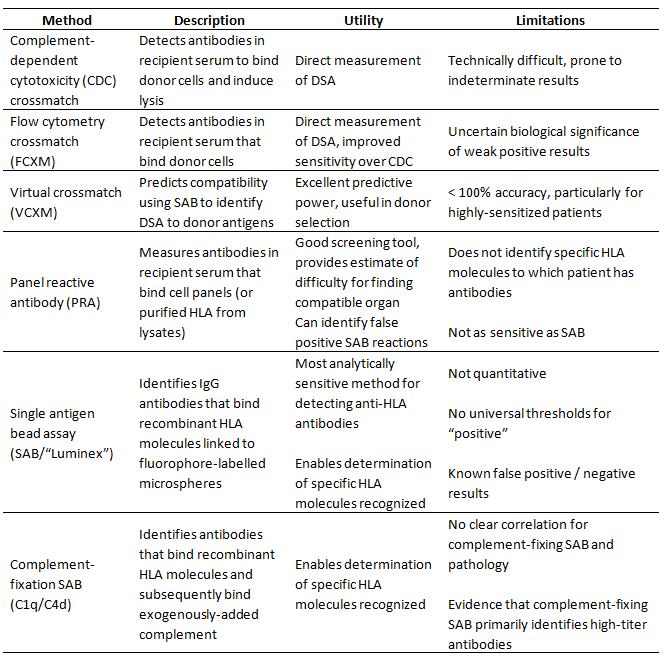
While antibodies are capable of recognizing allogeneic HLA molecules as foreign, there are significant differences between humoral and cellular alloreactivity. The most significant difference may be the limited extent of antigenic allotypic polymorphism recognized by antibodies. While the majority of the 1000s of different HLA alleles are capable of being recognized as foreign by allogeneic T cells, serologic reactivity is limited primarily to 139 recognized serologic antigens (Table 1) [4]. Furthermore, alloantibodies against HLA do not develop in the absence of sensitization [14], unlike T cell-mediated alloreactive responses which are robust even in the absence of prior sensitization. Thus, while preventing development of alloantibodies requires continual immune suppression, peri-transplant hyperacute AMR is theoretically avoidable through pre-transplant histocompatibility testing. Recognizing the significance of antibody-mediated rejection in solid organ transplantation, the histocompatibility field has developed increasingly sensitive and specific methods for HLA genotyping and identification of alloantibodies (Table 2). These advances have contributed to increased understanding of the immune-mediated processes causing lung allograft rejection and chronic dysfunction.
Importance of Pre-Existing Anti-HLA Antibodies and the Crossmatch for Donor Selection
Despite the evidence for pre-existing anti-HLA antibodies as mediators of hyperacute rejection in kidney and heart transplantation, pre-transplant assessment of histocompatibility for patients undergoing lung transplantation was not routinely performed in the early days of lung transplantation. A lack of enthusiasm for histocompatibility testing for lung transplantation was likely based on multiple factors. Scepticism of the clinical accuracy and utility of CDC crossmatching in thoracic transplantation and the relative efficacy of post-transplant immunosuppression [15] diminished the presumed importance of histocompatibility testing. Patients awaiting lung transplant were less likely to have been exposed to blood transfusion than heart or kidney transplant patients, reducing the likelihood of sensitization against alloantigens, and limitations in donor availability and cold ischemic time requirements made prospective crossmatch testing for recipient selection a seemingly dispensable luxury. However, as the numbers of lung transplants performed increased, cases where anti-HLA antibodies impacted clinical outcomes became apparent. A series of case reports of hyperacute rejection linked to the presence of alloantibodies [16–18] clearly demonstrated risk associated with pre-existing anti-HLA antibodies.
It is noteworthy that in two of these cases hyperacute rejection occurred in the context of a negative CDC crossmatch [17,18]. However, one of these cases [17] had a positive retrospective flow cytometry-based crossmatch, suggesting that the improved analytic sensitivity of flow cytometry could improve histocompatibility testing accuracy and clinical utility. To this end, examination of flow cytometry crossmatch (FCXM) testing in a series of 92 lung transplants demonstrated significant risk for hyperacute rejection associated with FCXM, with 3/6 FCXM positive transplants resulting in severe hyperacute rejection compared to only 4/86 FCXM negative transplants [19]. These studies identified the potential hazard posed by pre-existing anti-HLA antibodies and underscored the importance of crossmatch testing.
Table 2 Histocompatibility testing methods.
While it was evident that sensitization against allogeneic HLA posed risk for hyperacute rejection, it was unclear whether there was correlation between the quantitative degrees of sensitization described as panel reactive antibody (PRA) and relative risk. This was and remains an important question, as requirements for prospective crossmatch testing could significantly limit access to organs, particularly in thoracic transplantation. An early retrospective study of 247 lung transplant recipients found that only 8.5% had evidence of moderate or high-levels of sensitization (PRA ≥ 10%), only 14.3% of these patients had positive retrospective CDC crossmatches, and that neither PRA positive nor CDC crossmatch positive patients were at increased risk for acute rejection or BOS [20]. A subsequent retrospective study of 200 lung transplant patients tested by CDC panels identified a similar rate of sensitization (9% with PRA ≥ 10%), but indicated that HLA-sensitized patients had increased post-transplant ventilator requirements and were at increased risk for BOS [21]. Analysis of a larger cohort (n = 656) demonstrated rates of sensitization consistent with previous reports (15.4% with PRA > 0%, 5.6% with PRA ≥ 10%), but found that the 3% of patients with PRA ≥ 25% were at significant risk for decreased 30 day and 2 year survival after transplantation [22]. Decreased survival was further predicted in the highly-sensitized group by crossmatch testing, as patients with PRA ≥ 25% were more likely to have a positive CDC crossmatch (30% VS. 2.7%) and 4/6 high PRA patients with positive CDC crossmatch died within 30 days after transplantation compared to only 2/14 high PRA patients with negative crossmatch results. The correlation between a high degree of sensitization and poor outcomes was confirmed in a retrospective analysis of UNET data of 12,751 patients transplanted between 1987 and 2005, where patients with PRA ≥ 25% were at increased risk for death at 30 days, 1 year, 3 years, and 5 years post-transplant [23]. Interestingly, the effect of HLA sensitization was lost with subset analysis of patients transplanted between 1998 and 2005, the era after recognition of the potential danger for hyperactue rejection and implementation of routine pre-transplant histocompatibility testing.
Association between HLA sensitization, crossmatch incompatibility, and poor outcomes suggested that the more HLA antigens to which a patient developed antibodies against (higher PRA) increased the likelihood to be crossmatch positive against a potential donor, which was associated with the highest risk for hyperacute rejection. However, detection of anti-HLA antibodies by cell panels, whether via CDC or flow cytometry methods, did not facilitate precise determination of the specific HLA antigens against which the patient has antibodies. As newer methods for detecting anti-HLA antibodies were developed it became possible to identify specific HLA molecules to which patients were sensitized, enabling avoidance of donors with incompatible HLA. The applicability of the virtual crossmatch (VCXM) approach using this more specific information was first demonstrated in a retrospective single-center analysis, where identification of DSA in 16/341 patients predicted increased risk for BOS and mortality [24]. More importantly, the study demonstrated that the 12 patients with non-DSA HLA antibodies were not at increased risk for adverse events. This revealed that while overall sensitization against HLA presented increased challenge to identify an immunologically compatible donor, avoidance of DSA, via prospective cellular crossmatching or VCXM, is the critical challenge to prevent hyperacute rejection.
Single-Antigen Bead Assay (SAB) Screening for Anti-HLA Antibodies and the Virtual Crossmatch
Specific identification of anti-HLA antibodies was revolutionized by development of multiplexed single-antigen bead assays (SAB). SAB uses recombinant HLA molecules affixed to fluorophore barcoded microbeads on the Luminex platform to enable sensitive detection of antibodies against individual specific HLA molecules [25,26]. The increased analytic sensitivity of SAB is potentially clinically relevant, as retrospective analysis of lung transplants revealed low-level anti-HLA antibodies in 15-84% of patients [27,28,29,30,31,32], higher rates than demonstrated using CDC or flow cytometry-based PRA methods [20,21,22,24].
The increased analytic sensitivity of SAB has generated questions of the clinical relevance of identification of “weak” antibodies, referring to antibodies with low mean fluorescence intensity (MFI) values that may or may not be detectable by cell-based crossmatch. MFI represents the amount of fluorophore-labelled anti-IgG in complex with antibody-bound HLA-conjugated microbead. While there is some correlation between MFI and anti-HLA antibody titer, MFI is not an accurate quantitative measure of antibody concentration [33,34]; SAB is susceptible to interference, saturation, and prozone effects that preclude using MFI as a precise measurement. This, along with often arbitrary selection of MFI thresholds for identification of “positive” SAB results, has resulted in conflicting reports of the clinical significance of anti-HLA antibodies identified in pre-transplant serum by SAB. Studies using low MFI thresholds (1000–1200) indicate no negative effect for pre-existing DSA on lung transplant outcomes [31,32]. Conversely, pre-transplant DSA with MFI values > 3000 were associated with increased risk for AMR following lung transplantation [28]. Notably, this study reported a lack of effect of DSA with MFI values of 1000–3000, clearly demonstrating the importance of selecting MFI threshold values for identifying anti-HLA antibodies. This MFI threshold effect may explain suggested differences between pre-transplant DSA against class I and class II HLA associated with increased risk for BOS and decreased survival [27]. This study indicated that DSA against class II HLA, but not class I, was associated with increased risk for BOS and decreased survival, though there was significant difference in the MFI values for anti-class I (1048 ± 256) and anti-class II (2175 ± 597) DSA, precluding definitive assessment of differences in biological impacts between antibodies against class I or class II HLA.
While there is room for debate regarding MFI thresholds used for identifying potentially clinically-relevant anti-HLA antibodies, there is clear benefit to SAB and the VCXM approach to organ allocation. This highly-multiplexed flow cytometry approach of SAB permits a more refined assessment of HLA sensitization, where rather than a global likelihood for incompatibility estimated by PRA the effect of presence or absence of specific DSA can be evaluated [35]. Avoidance of DSA by VCXM can result in lung transplant outcomes equivalent to transplants with negative prospective crossmatch testing [36]. Thus, similar to heart and kidney transplantation, VCXM supported by SAB enables allocation of lungs with increased efficiency, facilitating transplantation of highly-sensitized patients [37–39] and enabling regional and national organ sharing [40]. However, VCXM accuracy can be compromised by incomplete HLA typing information, suboptimal MFI thresholds for SAB analysis [33,35,41], and technical limitations to SAB assays that cause false positive or false negative results [34,42–44]. In kidney transplantation populations, inaccuracies in VCXM prediction of immunologic compatibility are concentrated among highly-sensitized patients [38,44,45]. This suggests that for some patients, prospective cell-based crossmatch testing may be necessary to minimize risk (Figure 1).
Recognition of the pathogenic potential of anti-HLA antibodies in lung transplantation suggests that not only is it beneficial to avoid pre-existing humoral immunity against donor HLA antigens, but that DSA present after transplant may be indicative of rejection. Studies before the addition of SAB to the histocompatibility testing repertoire demonstrated an association with de novo development of anti-HLA antibodies with BOS, allograft rejection, and death [46–49]. While these reports are limited in the ability to specifically identify DSA, they all provide evidence for development of anti-HLA antibodies after transplantation preceding symptomatic evidence of rejection. In the cases where DSA could be determined, it was noted that 50-89% of these antibodies were against class II HLA antigens, suggesting a possible differential effect between class I and class II HLA in stimulating humoral immune responses mediating rejection [48,49]. These early studies present a compelling argument for a pathologic process where development of anti-donor humoral immune responses leads to lung allograft dysfunction.
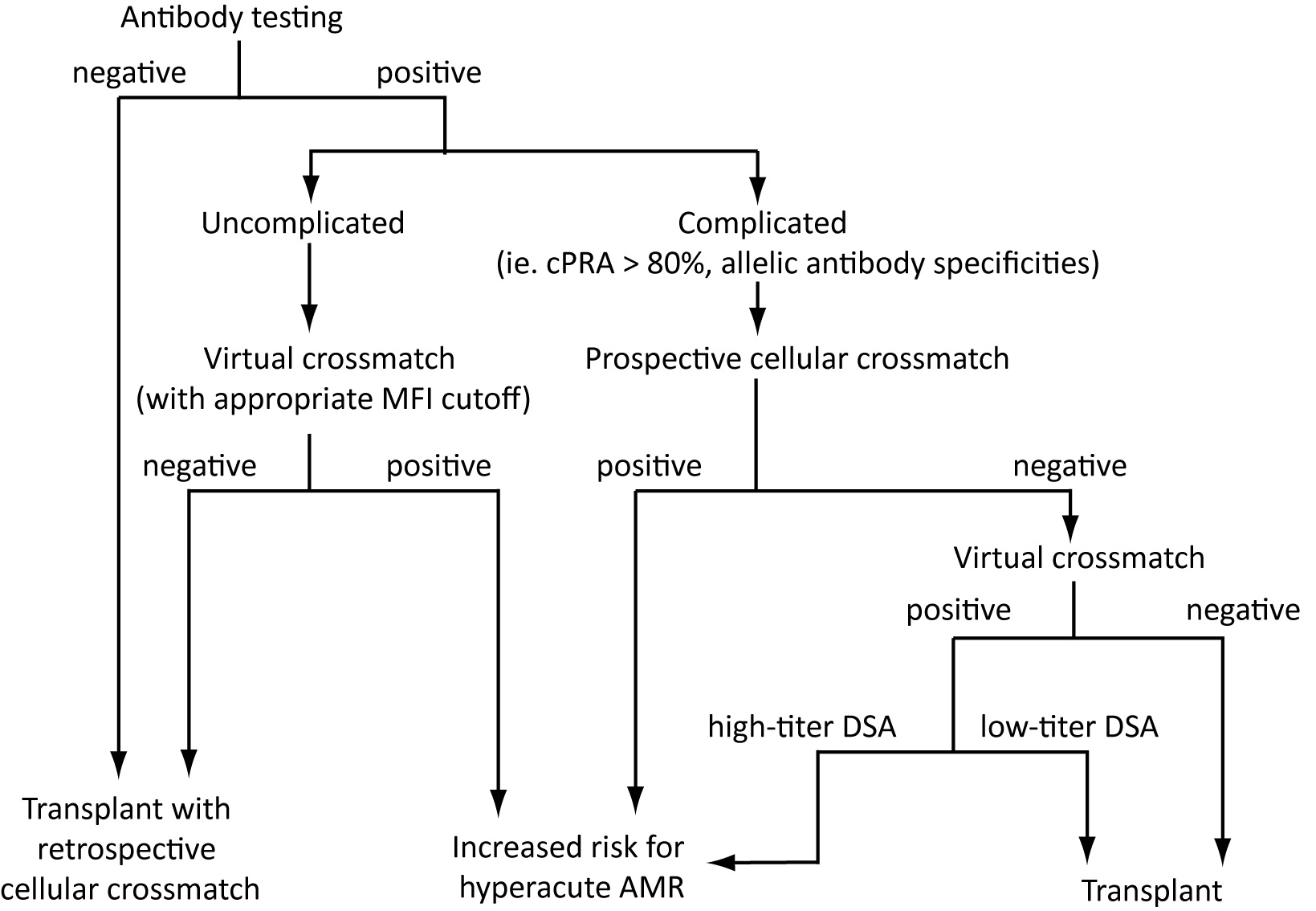
Figure 1 Algorithm for laboratory evaluation of donor compatibility. Incorporation of donor HLA typing, recipient alloantibody profiles and cellular crossmatch promotes optimal donor selection. SAB immunoassay enables accurate prediction of compatibility via VCXM, reducing dependence on prospective cellular CXM. However, patients with “complicated” alloantibody profiles may require prospective cellular crossmatch to evaluate compatibility due to increased risk of false negative SAB results or the presence of allele-specific anti-HLA antibodies where standard donor HLA typing may not provide sufficient information for VCXM. Patients determined to be at higher risk for hyperacute AMR may still be transplanted depending on medical urgency, and program-specific factors such as experience managing high-risk patients and experience with peri-transplant desensitization therapy.
Consequences of Donor-Specific Anti-HLA Antibodies Developing after Transplant
Given the demonstrations for the significance of DSA as opposed to non-DSA anti-HLA antibodies in pre-transplant testing, it is similarly important to specifically evaluate DSA in the post-transplant setting. Multiple studies have demonstrated high rates of DSA detectable by SAB, ranging from 10-61% of patients within 1 year following lung transplant [31,50,51,52,53]. DSA formation becomes more prevalent with increased time, as longer-term follow-up studies demonstrate DSA formation in 20-56% of patients [29,32,36,50,54,55,56,57]. Mean time to development of DSA varied among these studies, ranging from 52 days–40 months post-transplant. Interestingly, a majority of studies indicate no effect for non-DSA pre-transplant HLA antibodies on post-transplant DSA formation [28,31,36,54,56]. Reports to the contrary exist [50,51], though in at least one the authors do not explicitly rule out the possibility for DSA among pre-transplant anti-HLA antibodies [50]. Differences between reports in the frequency of patients with reported DSA formation and time post-transplant to develop detectable DSA are likely attributable to multiple factors including technical aspects of SAB testing (MFI threshold, etc.) as well as clinical variables. Regardless, these reports provide a consistent description of a high prevalence of de novo DSA formation after lung transplantation.
Similar to the consensus that generation of DSA is a common event after lung transplantation, studies of post-transplant humoral immunity consistently indicate that post-transplant DSA are associated with rejection and poor outcomes. Detection of DSA in post-transplant serum by SAB is associated with significantly increased risk for antibody-mediated processes of BOS [29,52,55], AMR [56,57,58], and CLAD [31,36,53,58]. These studies estimate hazard ratios of 1.6–6.6 for these modes of allograft dysfunction among patients with DSA, demonstrating the clinical significance of DSA formation. All of these mechanisms contribute to the increased post-transplant mortality of patients developing DSA [50–53,55]. Notably, longitudinal assessments of post-transplant alloantibodies have demonstrated that DSA can be detected in serum more than 250 days before the onset of clinically symptomatic allograft 31,52). This suggests that post-transplant DSA monitoring may enable early detection and possibly intervention in pathogenic processes contributing to lung allograft rejection. Several of these studies noted a disproportionate amount of DSA specific for class II HLA (50%–91%) compared to DSA recognizing class I antigens (24%–88%) [31,36,51,52,53,54,55,56]. Among DSA against class II antigens, antibodies against HLA-DQ predominated, present in 56-91% of patients [31,51,53,55,56]. It is unclear whether this reflects differential immunogenicity between HLA or if there are pathologic implications.
Implementation of routine monitoring protocols to detect post-transplant DSA requires considering criteria for defining antibodies with pathogenic potential. MFI values can be informative, though the caveats discussed in pre-transplant testing apply to post-transplant testing as well. Most reports demonstrating post-transplant complications associated with DSA utilize MFI thresholds of 1000–2000 [29,31,36,51,52,53,56,58,59], similar to those from pre-transplant studies predicting cellular crossmatch incompatibility. Studies directly examining MFI thresholds in post-transplant DSA detection indicate a relationship between increasing MFI and association with lung allograft dysfunction; DSA with MFI > 3000 were more predictive of BOS, CLAD, and mortality than DSA with MFI values between 1000–3000 [29,53]. This correlation between MFI values for antibodies in serum samples and allograft pathology is corroborated by the demonstration that DSA with higher MFI values (>6500) were most predictive of DSA that could be detected in lung biopsy eluates [60]. This suggests that pathogenic potential is related to the amount of DSA present, though MFI is an imperfect measure of antibody concentration.
Consideration has been given to other potential factors beyond MFI to differentiate pathogenic DSA that should prompt therapeutic intervention compared to those that may not pose proximal danger to the allograft. Studies in kidney and heart transplants have demonstrated some utility in measuring the ability of anti-HLA antibodies in SAB assays to bind complement components. These assays measure the presence of C4d or C1q on HLA-antibody-bound microbeads after addition of exogenous complement [61,62]. While there is correlation between C4d- and C1q-binding DSA and rejection in kidney and heart transplant patients, it has become clear that these assays preferentially detect high-titer antibodies [34,63,64]. This correlation between standard SAB MFI and complement-binding assays is similarly seen in studies of alloantibodies after lung transplant, where DSA with high MFI values (>4000) are also most associated with positive detection using the C1q binding assay [29,60]. Thus, complement-binding assays may be considered a method to identify high-titer DSA, though studies directly comparing titration of standard SAB with complement-binding assays suggest that serial dilution of serum and SAB may be more robust and informative for directing immunosuppressive therapy aimed at reducing antibody-mediated rejection.
Monitoring Anti-HLA Antibodies to Direct Post-Transplant Therapy
The correlation between detection of DSA and antibody-mediated lung allograft dysfunction suggests that evaluation of DSA may measure efficacy of therapies aimed at suppressing rejection. DSA are not only predictive or diagnostic for peri-transplant hyperacute rejection as well as post-transplant complications BOS, AMR, and CLAD, they also represent a pathogenic mechanism. While it is beyond the scope of this review to evaluate the clinical impact of therapeutic approaches to management of lung allograft rejection, it is important to consider how histocompatibility testing can be used to objectively assess outcomes.
In the peri-transplant setting, the problem that exists is one of limited organ availability in the setting of medically-urgent need. Particularly, the question is whether it is better to transplant with an immunologically-incompatible organ or to wait for a more compatible match. To address this, several studies have been performed evaluating peri-operative therapies aimed at reducing alloantibodies and avoiding hyperacute rejection. Given the association between cellular or virtual crossmatch and hyperacute rejection, it is attractive to consider conversion from a positive crossmatch result to negative as evidence of successful desensitization. Studies of different pre-transplant desensitization approaches have demonstrated variable efficacy in reducing anti-HLA antibodies detectable by SAB [59,65,66,67,68]. However, multiple studies have indicated that peri-transplant therapy resulting in conversion of cellular or virtual crossmatch (using appropriate MFI thresholds) from positive to negative are predictive of superior outcomes after transplantation of HLA-incompatible 6567).
Measurable reductions in circulating DSA detected by SAB are similarly associated with improved outcomes in response to treatment for AMR. Patients with measurable reductions in DSA MFI following therapy had better outcomes, reduced incidence of BOS, and improved survival compared to similarly treated patients with persistent DSA [56,66,68,69]. These data suggest that measurement of DSA may be an effective objective marker for treatment efficacy. Inclusion of laboratory evaluation of DSA should be an important aspect for trials of novel therapeutic approaches, providing empiric data to support clinical findings.
Beyond HLA
While antibodies to HLA are the principle mediators of antibody-mediated rejection of solid organ allografts, they are by no means the exclusive cause. However, it is becoming increasingly evident that immune responses against non-HLA antigens may be involved in antibody-mediated processes. In lung transplantation, antibodies against non-polymorphic autoantigens collagen (I and V) and K-α1 tubulin have been associated with graft rejection and BOS [57,69,70,71,72,73]. More studies are needed to evaluate the prevalence and risk associated with antibodies against non-HLA antigens, as well as to evaluate the role of pre- and post-transplant laboratory testing for providing useful diagnostic and prognostic information.
Conclusions
Lung transplantation has become an increasingly utilized and effective treatment for end-stage lung disease. Technical improvements in histocompatibility testing, including adaptation of flow cytometry and implementation of highly-sensitive multiplexed immunoassays for anti-HLA antibodies have supported continued progress in understanding immune-mediated complications after transplantation. Current histocompatibility testing methods not only support investigative work, but have significant clinical utility for directing pre- and post-transplant patient management. Significant evidence exists to support the use of VCXM and prospective crossmatch when necessary to direct organ allocation to avoid transplantation across a DSA barrier. Pre-transplant testing can aid in avoiding hyperacute rejection and promote maximal utility of donor organs. Likewise, significant evidence supports the use of flow cytometry and SAB assays to monitor DSA development after transplantation. Development of DSA is associated with significantly increased risk for AMR, BOS, CLAD, and mortality. While the precise temporal relationship between DSA emergence and these morbidities is unclear, it is evident that DSA formation precedes these events. This suggests that detection of DSA prior to onset of clinical symptoms may offer an opportunity for therapeutic intervention. Further studies, supported by histocompatibility laboratory testing as one objective measure of efficacy, are needed to evaluate novel therapeutic approaches and continue to improve lung transplantation outcomes.
Competing Interests
The author has declared that no competing interests exist.
References
- Valapour M, Skeans MA, Smith JM, Edwards LB, Cherikh WS, Callahan ER, et al. Lung. Am J Transplant. 2016;16(S2):141-68. [CrossRef] [Google scholar] [PubMed]
- Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451-62. [CrossRef] [Google scholar] [PubMed]
- Rydberg L. ABO-incompatibility in solid organ transplantation. Transfusion Med. 2001;11:325-42. [CrossRef] [Google scholar] [PubMed]
- Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SG. The IPD and IMGT/HLA database: allele variant databases. Nucl Acid Res. 2015;43:D423-31. [CrossRef] [Google scholar] [PubMed]
- Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nat Rev Immunol. 2007;7:942-53. [CrossRef] [Google scholar] [PubMed]
- Orens JB, Garrity ER, Jr. General overview of lung transplantation and review of organ allocation. Proc Am Thoracic Soc. 2009;6:13-9. [CrossRef] [Google scholar] [PubMed]
- Adegunsoye A, Strek ME, Garrity E, Guzy R, Bag R. Comprehensive Care of the Lung Transplant Patient. Chest. 2016;10.1016/j.chest.2016.10.001. [CrossRef] [Google scholar] [PubMed]
- Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, Berry GJ, et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2016;35:397-406. [CrossRef] [Google scholar] [PubMed]
- Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807-17. [CrossRef] [Google scholar] [PubMed]
- Colvin MM, Cook JL, Chang P, Francis G, Hsu DT, Kiernan MS, et al. Antibody-mediated rejection in cardiac transplantation: emerging knowledge in diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2015;131:1608-39. [CrossRef] [Google scholar] [PubMed]
- Wallace WD, LI N, Andersen CB, Arrossi AV, Askar M, Berry GJ, et al. Banff study of pathologic changes in lung allograft biopsy specimens with donor-specific anrtibodies. J Heart Lung Transplant. 2016;35:40-8. [CrossRef] [Google scholar] [PubMed]
- Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, Wilson KC, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Euro Respir J. 2014;44:1479-503. [CrossRef] [Google scholar] [PubMed]
- Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127-33. [CrossRef] [Google scholar] [PubMed]
- Scornik JC, Kriesche HU. Human leukocyte antigen sensitization after transplant loss: timing of antibody detection and implications for prevention. Human Immunol. 2011;72:398-401. [CrossRef] [Google scholar] [PubMed]
- Trento A, Hardesty RL, Griffith BP, Zerbe T, Kormos RL, Bahnson HT. Role of the antibody to vascular endothelial cells in hyperacute rejection in patients undergoing cardiac transplantation. J Thoracic Cardiovascular Surg. 1988;95:37-41. [Google scholar]
- Frost AE, Jammal CT, Cagle PT. Hyperacute rejection following lung transplantation. Chest. 1996;110:559-62. [CrossRef] [Google scholar] [PubMed]
- Choi JK, Kearns J, Palevsky HI, Montone KT, Kaiser LR, Zmijewski CM, et al. Hyperacute rejection of a pulmonary allograft. Immediate clinical and pathologic findings. Am J Resp Crit Care Med. 1999;160:1015-8. [CrossRef] [Google scholar] [PubMed]
- Bittner HB, Dunitz J, Hertz M, Bolman MR, 3rd, Park SJ. Hyperacute rejection in single lung transplantation—case report of successful management by means of plasmapheresis and antithymocyte globulin treatment. Transplantation. 2001;71:649-51. [CrossRef] [Google scholar] [PubMed]
- Scornik JC, Zander DS, Baz MA, Donnelly WH, Staples ED. Susceptibility of lung transplants to preformed donor-specific HLA antibodies as detected by flow cytometry. Transplantation. 1999;68:1542-6. [CrossRef] [Google scholar] [PubMed]
- Gammie JS, Pham SM, Colson YL, Kawai A, Keenan RJ, Weyant RJ, et al. Influence of panel-reactive antibody on survival and rejection after lung transplantation. J Heart Lung Transplant. 1997;16:408-15. [Google scholar]
- Lau CL, Palmer SM, Posther KE, Howell DN, Reinsmoen NL, Massey HT, et al. Influence of panel-reactive antibodies on posttransplant outcomes in lung transplant recipients. Ann Thorac Surg. 2000;69:1520-4. [CrossRef] [Google scholar] [PubMed]
- Hadjiliadis D, Chaparro C, Reinsmoen NL, Gutierrez C, Singer LG, Steele MP, et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant 2005;24:S249-54. [CrossRef] [Google scholar] [PubMed]
- Shah AS, Nwakanma L, Simpkins C, Williams J, Chang DC, Conte JV. Pretransplant panel reactive antibodies in human lung transplantation: an analysis of over 10,000 patients. Ann Thorac Surg. 2008;85:1919-24. [CrossRef] [Google scholar] [PubMed]
- Appel JZ, 3rd, Hartwig MG, Cantu E, 3rd, Palmer SM, Reinsmoen NL, Davis RD. Role of flow cytometry to define unacceptable HLA antigens in lung transplant recipients with HLA-specific antibodies. Transplantation. 2006;81:1049-57. [CrossRef] [Google scholar] [PubMed]
- Pei R, Lee J, Chen T, Rojo S, Terasaki PI. Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Human Immunol. 1999;60:1293-302. [CrossRef] [Google scholar] [PubMed]
- Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75:43-9. [CrossRef] [Google scholar] [PubMed]
- Brugiere O, Suberbielle C, Thabut G, Lhuillier E, Dauriat G, Metivier AC, et al. Lung transplantation in patients with pretransplantation donor-specific antibodies detected by Luminex assay. Transplantation. 2013;95:761-5. [CrossRef] [Google scholar] [PubMed]
- Kim M, Townsend KR, Wood IG, Boukedes S, Guleria I, Gabardi S, et al. Impact of pretransplant anti-HLA antibodies on outcomes in lung transplant candidates. Am J Respir Crit Care Med. 2014;189:1234-9. [CrossRef] [Google scholar] [PubMed]
- Kauke T, Kneidinger N, Martin B, Dick A, Schneider C, Schramm R, et al. Bronchiolitis obliterans syndrome due to donor-specific HLA-antibodies. Tissue Antigens. 2015;86:178-85. [CrossRef] [Google scholar] [PubMed]
- Chin N, Paraskeva M, Paul E, Cantwell L, Levvey B, Williams T, et al. Comparative analysis of how immune sensitization is defined prior to lung transplantation. Human Immunol. 2015;76:711-6. [CrossRef] [Google scholar] [PubMed]
- Tikkanen JM, Singer LG, Kim SJ, Li Y, Binnie M, Chaparro C, et al. De Novo DQ Donor-Specific Antibodies Are Associated with Chronic Lung Allograft Dysfunction after Lung Transplantation. Am J Respir Crit Care Med. 2016;194:596-606. [CrossRef] [Google scholar] [PubMed]
- Zazueta OE, Preston SE, Moniodis A, Fried S, Kim M, Townsend K, et al. The Presence of Pretransplant HLA Antibodies Does Not Impact the Development of Chronic Lung Allograft Dysfunction or CLAD Related Death. Transplantation. 2016; doi:10.1097/TP.0000000000001494. [CrossRef] [Google scholar] [PubMed]
- 33.Liu C, Wetter L, Pang S, Phelan DL, Mohanakumar T, Morris GP. Cutoff values and data handling for solid-phase testing for antibodies to HLA: effects on listing unacceptable antigens for thoracic organ transplantation. Human Immunol. 2012;73:597-604. [CrossRef] [Google scholar] [PubMed]
- Tambur AR, Herrera ND, Haarberg KM, Cusick MF, Gordon RA, Leventhal JR, et al. Assessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am J Transplant. 2015;15:2421-30. [CrossRef] [Google scholar] [PubMed]
- Tambur AR, Ramon DS, Kaufman DB, Friedewald J, Luo X, Ho B, et al. Perception versus reality?: Virtual crossmatch—how to overcome some of the technical and logistic limitations. Am J Transplant. 2009;9:1886-93. [CrossRef] [Google scholar] [PubMed]
- Bosanquet JP, Witt CA, Bemiss BC, Byers DE, Yusen RD, Patterson AG, et al. The impact of pre-transplant allosensitization on outcomes after lung transplantation. J Heart Lung Transplant. 2015;34:1415-22. [CrossRef] [Google scholar] [PubMed]
- Bingaman AW, Murphey CL, Palma-Vargas J, Wright F. A virtual crossmatch protocol significantly increases access of highly sensitized patients to deceased donor kidney transplantation. Transplantation. 2008;86:1864-8. [CrossRef] [Google scholar] [PubMed]
- Baxter-Lowe LA, Kucheryavaya A, Tyan D, Reinsmoen N. CPRA for allocation of kidneys in the US: More candidates ≥98% CPRA, lower positive crossmatch rates and improved transplant rates for sensitized patients. Human Immunol. 2016;77:395-402. [CrossRef] [Google scholar] [PubMed]
- Reinsmoen NL, Patel J, Mirocha J, Lai CH, Naim M, Ong G, et al. Optimizing transplantation of sensitized heart candidates using 4 antibody detection assays to prioritize the assignment of unacceptable antigens. J Heart Lung Transplant. 2016;35:165-72. [CrossRef] [Google scholar] [PubMed]
- Baxter-Lowe LA, Cecka M, Kamoun M, Sinacore J, Melcher ML. Center-defined unacceptable HLA antigens facilitate transplants for sensitized patients in a multi-center kidney exchange program. Am J Transplant. 2014;14:1592-8. [CrossRef] [Google scholar] [PubMed]
- Ellis TM, Schiller JJ, Roza AM, Cronin DC, Shames BD, Johnson CP. Diagnostic accuracy of solid phase HLA antibody assays for prediction of crossmatch strength. Human Immunol. 2012;73:706-10. [CrossRef] [Google scholar] [PubMed]
- Zachary AA, Lucas DP, Detrick B, Leffell MS. Naturally occurring interference in Luminex assays for HLA-specific antibodies: characteristics and resolution. Human Immunol. 2009;70:496-501. [CrossRef] [Google scholar] [PubMed]
- Schwaiger E, Wahrmann M, Bond G, Eskandary F, Bohmig GA. Complement component C3 activation: the leading cause of the prozone phenomenon affecting HLA antibody detection on single-antigen beads. Transplantation. 2014;97:1279-85. [CrossRef] [Google scholar] [PubMed]
- Jani V, Ingulli E, Mekeel K, Morris GP. Root cause analysis of limitations of virtual crossmatch for kidney allocation to highly-sensitized patients. Human Immunol. 2016; doi: 10.1016/j.humimm.2016.11.003. [CrossRef] [Google scholar]
- Tambur AR, Haarberg KM, Friedewald JJ, Leventhal JR, Cusick MF, Jaramillo A, et al. Unintended Consequences of the New National Kidney Allocation Policy in the United States. Am J Transplant. 2015;15:2465-9. [CrossRef] [Google scholar] [PubMed]
- Sundaresan S, Mohanakumar T, Smith MA, Trulock EP, Lynch J, Phelan D, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65:648-53. [CrossRef] [Google scholar] [PubMed]
- Jaramillo A, Smith MA, Phelan D, Sundaresan S, Trulock EP, Lynch JP, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67:1155-61. [CrossRef] [Google scholar] [PubMed]
- Palmer SM, Davis RD, Hadjiliadis D, Hertz MI, Howell DN, Ward FE, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74:799-804. [CrossRef] [Google scholar] [PubMed]
- Girnita AL, McCurry KR, Iacono AT, Duquesnoy R, Corcoran TE, Awad M, et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. 2004;23:1135-41. [CrossRef] [Google scholar] [PubMed]
- Snyder LD, Wang Z, Chen DF, Reinsmoen NL, Finlen-Copeland CA, Davis WA, et al. Implications for human leukocyte antigen antibodies after lung transplantation: a 10-year experience in 441 patients. Chest. 2013;144:226-33. [CrossRef] [Google scholar] [PubMed]
- Ius F, Sommer W, Tudorache I, Kuhn C, Avsar M, Siemeni T, et al. Early donor-specific antibodies in lung transplantation: risk factors and impact on survival. J Heart Lung Transplant. 2014;33:1255-63. [CrossRef] [Google scholar] [PubMed]
- Morrell MR, Pilewski JM, Gries CJ, Pipeling MR, Crespo MM, Ensor CR, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. 2014;33:1288-94. [CrossRef] [Google scholar] [PubMed]
- Le Pavec J, Suberbielle C, Lamrani L, Feuillet S, Savale L, Dorfmuller P, et al. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant. 2016;35:1067-77. [CrossRef] [Google scholar] [PubMed]
- Hachem RR, Yusen RD, Meyers BF, Aloush AA, Mohanakumar T, Patterson GA, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973-80. [CrossRef] [Google scholar] [PubMed]
- Safavi S, Robinson DR, Soresi S, Carby M, Smith JD. De novo donor HLA-specific antibodies predict development of bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2014;33:1273-81. [CrossRef] [Google scholar] [PubMed]
- Witt CA, Gaut JP, Yusen RD, Byers DE, Iuppa JA, Bennett Bain K, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013;32:1034-40. [CrossRef] [Google scholar] [PubMed]
- Reinsmoen NL, Mirocha J, Ensor CR, Marrari M, Chaux G, Levine DJ, et al. A 3 Center Study Reveals New Insights into the Impact of NonHLA Antibodies On Lung Transplantation Outcome. Transplantation. 2016;doi:10.1097/TP.0000000000001389. [CrossRef] [Google scholar] [PubMed]
- Roux A, Bendib Le lan I, Holifanjaniaina S, Thomas KA, Hamid AM, et al. Antibody-mediated rejection in lung transplantation: clinical outcomes and donor-specific antibody characteristics. Am J Transplant. 2016;16:1216-28. [CrossRef] [Google scholar] [PubMed]
- Snyder LD, Gray AL, Reynolds JM, Arepally GM, Bedoya A, Hartwig MG, et al. Antibody desensitization therapy in highly sensitized lung transplant candidates. Am J Transplant. 2014;14:849-56. [CrossRef] [Google scholar] [PubMed]
- Visentin J, Chartier A, Massara L, Linares G, Guidicelli G, Blanchard E, et al. Lung intragraft donor-specific antibodies as a risk factor for graft loss. J Heart Lung Transplant. 2016;35:1418-26. [CrossRef] [Google scholar] [PubMed]
- Wahrmann M, Exner M, Haidbauer B, Schillinger M, Regele H, Kormoczi G, et al. [C4d]FlowPRA screening—A specific assay for selective detection of complement-activating anti-HLA alloantibodies. Human Immunol. 2005;66:526-34. [CrossRef] [Google scholar] [PubMed]
- Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Human Immunol. 2011;72:849-58. [CrossRef] [Google scholar] [PubMed]
- Crespo M, Torio A, Mas V, Redondo D, Perez-Saez MJ, Mir M, et al. Clinical relevance of pretransplant anti-HLA donor-specific antibodies: does C1q-fixation matter? Transplant Immunol. 2013;29:28-33. [CrossRef] [Google scholar] [PubMed]
- Schaub S, Honger G, Koller MT, Liwski R, Amico P. Determinants of C1q binding in the single antigen bead assay. Transplantation. 2014;98:387-93. [CrossRef] [Google scholar] [PubMed]
- Appel JZ, 3rd, Hartwig MG, Davis RD, Reinsmoen NL. Utility of peritransplant and rescue intravenous immunoglobulin and extracorporeal immunoadsorption in lung transplant recipients sensitized to HLA antigens. Human Immunol. 2005;66:378-86. [CrossRef] [Google scholar] [PubMed]
- Jackups R, Jr., Canter C, Sweet SC, Mohanakumar T, Morris GP. Measurement of donor-specific HLA antibodies following plasma exchange therapy predicts clinical outcome in pediatric heart and lung transplant recipients with antibody-mediated rejection. J Clin Apheresis. 2013;28:301-8. [CrossRef] [Google scholar] [PubMed]
- Tinckam KJ, Keshavjee S, Chaparro C, Barth D, Azad S, Binnie M, et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am J Transplant. 2015;15:417-26. [CrossRef] [Google scholar] [PubMed]
- Ius F, Sommer W, Tudorache I, Kuhn C, Avsar M, Siemeni T, et al. Preemptive treatment with therapeutic plasma exchange and rituximab for early donor-specific antibodies after lung transplantation. J Heart Lung Transplant. 2015;34:50-8. [CrossRef] [Google scholar] [PubMed]
- Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to K-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12:2164-71. [CrossRef] [Google scholar] [PubMed]
- Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487-94. [CrossRef] [Google scholar] [PubMed]
- Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624-31. [CrossRef] [Google scholar] [PubMed]
- Tiriveedhi V, Gautam B, Sarma NJ, Askar M, Budev M, Aloush A, et al. Pre-transplant antibodies to Kalpha1 tubulin and collagen-V in lung transplantation: clinical correlations. J Heart Lung Transplant. 2013;32:807-14. [CrossRef] [Google scholar] [PubMed]
- Fernandez R, Chiu S, Raparia K, Garcha P, Farver C, Budev M, et al. Humoral Human Lung Allograft Rejection by Tissue-Restricted Non-HLA Antibodies. Ann Thorac Surg. 2016;102:e339-41. [CrossRef] [Google scholar] [PubMed]



