The Relationship between Pneumocystis Infection in Animal and Human Hosts, and Climatological and Environmental Air Pollution Factors: A Systematic Review
Robert F. Miller 1,2,3,4,*![]() , Laurence Huang 5,6
, Laurence Huang 5,6![]() , Peter D. Walzer 7
, Peter D. Walzer 7![]()
- Centre for Clinical Research in Infection and Sexual Health, Institute for Global Health, University College London, London WC1E 6JB, UK
- Clinical Research Department, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK
- Bloomsbury Clinic, Mortimer Market Centre, Central & North West London NHS Foundation Trust, London WC1E 6JB, UK
- HIV Services, Royal Free London NHS Foundation Trust, London NW3 2QG, UK
- Division of Pulmonary and Critical Care Medicine, Zuckerberg San Francisco General Hospital and Trauma Center, University of California, San Francisco, CA 94110, USA
- HIV, Infectious Diseases, and Global Medicine Division, Zuckerberg San Francisco General Hospital and Trauma Center, University of California, San Francisco, San Francisco, CA 94110, USA
- Department of Internal Medicine, University of Cincinnati, Cincinnati, OH 45267, USA
* Correspondence: Robert F Miller![]()
Received: September 14, 2018 | Accepted: October 19, 2018 | Published: October 26, 2018
OBM Genetics 2018, Volume 2, Issue 4 doi: 10.21926/obm.genet.1804045
Academic Editors: Andrés Moya, Enrique J. Calderón, and Luis Delaye
Special Issue: Pneumocystis: A Model of Adaptive Coevolution
Recommended citation: Miller RF, Huang L, Walzer PD. The Relationship between Pneumocystis Infection in Animal and Human Hosts, and Climatological and Environmental Air Pollution Factors: A Systematic Review. OBM Genetics 2018;2(4):045; doi:10.21926/obm.genet.1804045.
© 2018 by the authors. This is an open access article distributed under the conditions of the Creative Commons by Attribution License, which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is correctly cited.
Abstract
Background: Over the past decade, there has been rising interest in the interaction of Pneumocystis with the environment. This interest has arisen in part from the demonstration that environmental factors have important effects on the viability and transmission of microbes, including Pneumocystis. Environmental factors include climatological factors such as temperature, humidity, and precipitation, and air pollution factors including carbon monoxide, nitrogen dioxide, sulfur dioxide, and particulate matter. Methods: We undertook a systematic review in order to identify environmental factors associated with Pneumocystis infection or PCP, and their effects on human and animal hosts. Results: The systematic review found evidence of associations between Pneumocystis infection in animal and human hosts, and climatological and air pollution factors. Data from human studies infers that rather than a seasonal association, presentation with PCP appears to be highest when the average temperature is between 10 and 20°C. There was evidence of an association with hospitalization with PCP and ambient air pollution factors, as well as evidence of an effect of air pollution on both systemic and bronchoscopic lavage fluid humoral responses to Pneumocystis. Interpretation of human studies was confounded by possible genetically-determined predisposition to, or protection from infection. Conclusions: This systematic review provides evidence of associations between Pneumocystis infection in both animal and human hosts, and climatological and environmental air pollution factors. This information may lead to an improved understanding of the conditions involved in transmission of Pneumocystis in both animal and human hosts. Such knowledge is critical to efforts aimed at prevention.
Keywords
Pneumocystis; season; temperature; climate; environment; air pollution; detection
1. Introduction
Pneumocystis jirovecii is a fungus that continues to be an important cause of pneumonia (PCP) in the immunocompromised host and a major cause of death in humans [1]. It is estimated that there are more than 400,000 annual cases of PCP worldwide, with over 52,000 deaths per year [2]. Knowledge of the basic biology of Pneumocystis has long been limited by of the lack of a reliable and reproducible method of in vitro cultivation [3] but important insights have been gained from both animal and human studies. Pneumocystis organisms found in different hosts are morphologically indistinguishable but host species-specific [1]. Colonization of apparently healthy asymptomatic humans may provide a reservoir of P. jirovecii, and transmission of Pneumocystis to both susceptible and healthy persons may occur. However, the exact relationship is incompletely understood, and environmental reservoirs for the organism have also been suggested. Pneumocystis infection is acquired by inhalation, and the infective moiety is the cystic form [4] but the precise conditions for airborne transmission are unknown. Traditionally, PCP was thought to be the result of reactivation of latent infection acquired early in childhood, but molecular studies from PCP outbreaks demonstrate that PCP can also result from recent exposure in an at-risk host [5]. As a result, an improved understanding of the conditions involved in transmission is critical to efforts at prevention.
Over the past decade, there has been rising interest in the interaction of Pneumocystis with the environment. This interest has arisen in part from the demonstration that environmental factors have important effects on the viability and transmission of microbes, including Pneumocystis. Studies have demonstrated an association between environmental factors and the risk of PCP as well as specific antibody responses against P. jirovecii. The environmental factors can broadly be divided into two groups: a) climatological factors such as temperature, humidity, and precipitation; and b) air pollution factors including carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2) and particulate matter [6]. We undertook this systematic review in order to identify specific environmental factors associated with Pneumocystis infection or PCP, and their effects on human and animal hosts. Our goal was to comprehensively review the published literature on this topic in order to gain insights and advance our understanding of this important human pathogen.
2. Materials and Methods
We sought to identify publications describing associations between environmental factors and detection of Pneumocystis in humans and animals, and with development and presentation with PCP. We first reviewed English-language published articles of Pneumocystis and PCP and associated climatological and air pollution factors for the period 01 January 1960 to the present day (30 June 2018) using PubMed (US National Library of Medicine). The following search terms were used: Pneumocystis [Title] + English [Language], then Human [MeSH], then one of the following MeSH: season, climate, air pollution, environment, geography, humidity, or temperature. In addition, we reviewed the references within each publication for additional articles. Since the first reports of human immunodeficiency virus (HIV)-associated PCP in the early 1980s, most cases of PCP in the literature have been described in the context of HIV infection.
The scope of the present study was increased to include animals studied in the wild, in slaughterhouses, and in research laboratories. The same time period was used and the following search terms were used in the literature search: Pneumocystis [Title] + English [Language], then, Animal [MeSH], then one of the following MeSH: season, climate, air pollution, environment, geography, humidity, or temperature. Inclusion of these animal studies was done in order to enhance the findings from human studies and to provide important insights that add to our understanding of studies in humans.
Studies identified by the search strategy were divided into three groups:
Group 1: animal studies. Group 2: human studies of HIV-infected and uninfected patients with PCP divided based on geographic location. Group 3: human studies of HIV-infected patients with PCP associated with ambient air pollutants.
Where specific climatological information (i.e., temperature and humidity/precipitation) was not described in a specific publication we used World Weather Online [7]. In addition, as this is a systematic review of previously published work, Ethics Committee/IRB approval was not required from any of the three centers involved in this work.
3. Results
3.1 Literature Search
The PubMed literature search for the period 01 January 1960 through 30 June 2018 identified 74 unique full text articles. From these, 53 articles were excluded (after review of the full text by LH and RFM), as they were not relevant to the topic, or they were review articles that did not contain original data. In addition to the 21 remaining articles, 16 articles were identified by hand searching by the authors (LH and RFM). Additionally, the authors of some publications identified either by the MeSH search or via the “hand search” were contacted (by RFM) in order to obtain additional information not contained in their original published manuscript. Thus, in total 37 articles were identified and were included in the systematic review. Figure 1 shows the results of the literature search.
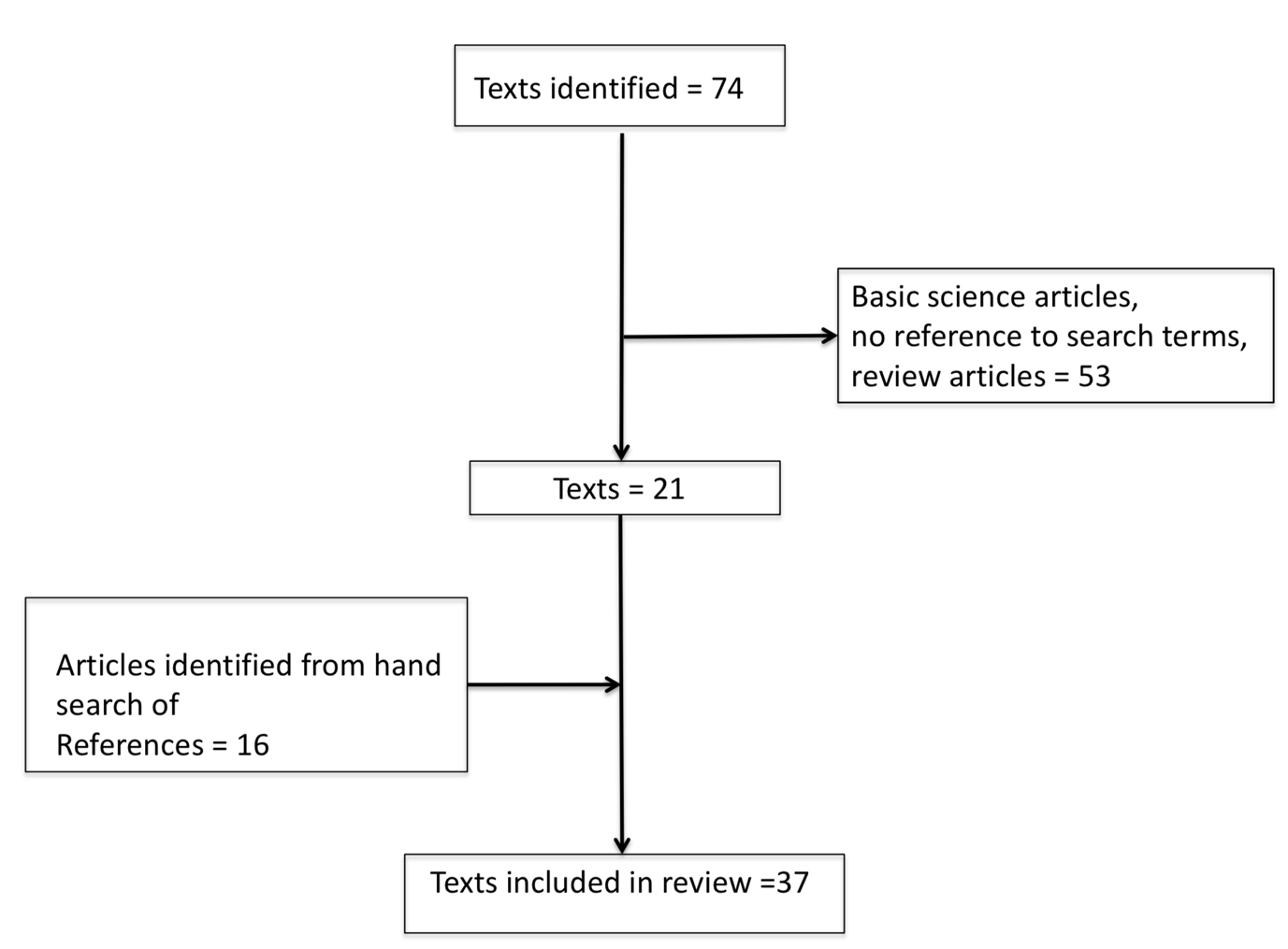
Figure 1 Results of literature search.
3.2 Animal Studies Showing Associations between Detection of Pneumocystis and Seasonal and Environmental Factors
Group 1. Eleven studies in animals (six from Europe, two from South America, two from Asia, and one from USA) were identified. Animal hosts were pigs (four studies), wild mouse species, shrew species, field voles, rats, hares, crab-eating macaques, and bats (each one study), (Table 1) [8,9,10,11,12,13,14,15,16,17,18]. Pneumocystis was detected in all of the animal species studied, using a range of techniques including Grocott silver staining, immunohistochemistry, and polymerase chain reaction (PCR). Using PCR, Pneumocystis was detected in as few as 4-5% of pigs in a slaughterhouse [17] to as many as 34.5% of crab-eating macaques living in a Primatology Center [12].
Table 1 Animal studies showing associations between detection of Pneumocystis spp and seasonal and environmental factors.

Key: PCR = polymerase chain reaction; ISH = in situ hybridization; PCV2 = porcine circovirus type 2; PRRSV = porcine reproductive and respiratory syndrome virus; TTSuV1, TTSuV2 = torque teno sus virus type 1 and 2.
The main findings were that in eight of the 11 studies there was a clear seasonal variation in detection of Pneumocystis among different hosts, in their natural surroundings [8,9,10,11,14,15,17,18]. In the study of macaques housed in a Primatology Center, detection was associated with mean precipitation, and in the study of rats in a laboratory facility, temperature and humidity had a clear influence on the predominance of different types of Pneumocystis. Finally, the study of bats in both captive and wild environments showed no association between detection of Pneumocystis and either temperature or humidity, but an association with smaller, crowded sedentary colonies at altitudes below 800m above sea level.
3.3 Human Studies Showing Associations between Pneumocystis and Seasonal and Environmental Factors
Group 2. Twenty-four studies in HIV-infected and uninfected patients with PCP were identified and were divided, based on geographic location. Seven studies were from USA, two from South America, 11 from Northern Europe, three from Southern Europe, and one from Australia, (Table 2) [6,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. These studies were a mixture of prospective and retrospective clinical or laboratory (including autopsy) studies and were national, multi-center or single center in their design. Two studies were done in infants, the remaining 22 studies were done in adults. Among the adult studies 19 included only HIV-infected adults, two included HIV-infected and uninfected adults, and one included HIV-uninfected adults with underlying rheumatologic conditions. In the two studies of infants, subjects were HIV-uninfected [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41].
The main findings were that in 18 studies in adults there was evidence of a seasonal and/or climatological association and presentation with PCP, or detection at autopsy and two studies showed no such association but noted clustering of cases of PCP by Zip Code, and another identified that time spent outdoors was associated with risk of PCP. In the remaining two studies there was no apparent seasonal or climatologic association with development of PCP. However, both studies showed an apparent ethnic predisposition, with patients of black African origin being at reduced risk of developing PCP compared to patients who were of Western origin (Table 2). Both studies in infants showed a seasonal variation either in detection of P. jirovecii at autopsy, or variation in antibody responses to Pneumocystis major surface antigen components.
Table 2 Human studies showing associations between Pneumocystis and seasonal and environmental factors.

Key: MACS = Multi-center AIDS cohort study; MSM = men who have sex with men; PCP = Pneumocystis jirovecii pneumonia; CDC = Centers for Disease Control and Prevention; ATHENA = AIDS Therapy Evaluation in the Netherlands; Msg = major surface glycoprotein; PCR = polymerase chain reaction; mt LSU rRNA = mitochondrial large subunit ribosomal RNA. *data from World Weather Online [7].
3.4 Human Studies Showing Associations between Pneumocystis and Ambient Air Pollution Factors
Group 3. Four studies (three from USA, one from Spain) reported the impact of ambient air pollution factors among HIV-infected adult patients hospitalized with PCP (Table 3) [6,40,42,43]. Of note two of these four studies are also included in Group 2 [6,42]. The San Francisco studies were single-center, prospective studies while the study from Spain was a national study.
The main findings were that one study showed elevated levels of NO2, PM10 and ozone in ambient air were associated with increased risk of hospitalization with PCP [40], and in another SO2, was associated with increased risk of hospitalization, but the risk was attenuated by elevated CO levels [6]. Two further studies that examined serologic responses in hospitalized patients with PCP, and showed elevated NO2, and PM10 were independently associated with impaired IgM responses to P. jirovecii major surface glycoprotein (Msg) constructs in serum [42], and that there was an impaired IgA response to P. jirovecii Msg in bronchoscopic lavage (BAL) fluid that was associated with increased ambient ozone exposure. Additionally, increased BAL fluid IgA responses were associated with increased ambient NO2 exposure [43] (Table 3).
Table 3 Human studies showing associations between Pneumocystis and ambient air pollution factors.

4. Discussion
We believe that the present study is the first systematic review of the relationship between Pneumocystis infection in its animal and human hosts, and the effects of climatological and air pollution factors in the environment on this relationship. This review found evidence of associations between Pneumocystis infection in animal and human hosts, and climatological and environmental air pollution factors, but the quality of evidence was poor and was limited by inconsistent and incomplete sampling methodology in both animal and human studies.
4.1 Non-Pneumocystis Fungi – Seasonal and Climatological Factors
Climatological factors, including temperature and humidity, are associated with variations in concentrations of ascomycetes, basidiomycetes, and other fungi in air [44,45,46,47,48]. A study from Porto Alegre, Southern Brazil, showed the highest detection of airborne fungal spores was in summer (December-February), when average minimum air temperatures are typically between 18 and 21°C [7]. The lowest rates of detection were in the autumn (March-May), when average minimum temperatures are typically between 12 and 17°C [45]. A second study from Fortaleza, North East Brazil, where the climate is hotter, reported seasonal variation in rates of detection of airborne fungi, (higher rates of detection were observed in January-April, and lower rates were recorded in July-October [46]. This city experiences a rainy season (February-July: rainfall 1883.5 mm, average temperature 26.8°C) and a dry season (August-January: rainfall 260 mm, average temperature 27.6°C) [46].
4.2 Pneumocystis - Seasonal Factors – Average Temperature
In the studies of Pneumocystis in animals studied in the wild, in slaughterhouses, and in research laboratories that were identified in this systematic review interpretation of the observed seasonal variation is confounded by the fact that none of the studies systematically sampled throughout the year, nor sampled in consecutive years, and none of the studies included “controls”. Thus, possible alternative explanations for the apparent seasonal variation must include closer animal-to-animal proximity within animal colonies, driven by climatological factors, and by season, for example by closer co-habitation during the mating season for an individual animal host. This potentially increases the chance for airborne or “other” animal-to-animal transmission, and thus likelihood of detection by opportunistic, or systematic sampling. In the studies of pigs, another factor influencing detection of Pneumocystis is the type of husbandry used in rearing the animals, as higher rates of detection were observed in semi-intensively farmed animals when compared with pigs who were intensively-farmed. This finding has been ascribed to less control of production variables in pigs reared semi-intensively [17]. Additionally, detection of Pneumocystis has been associated with underlying viral and/or bacterial infection in pigs. It has been suggested that these infections may act as potential “immune suppressants” thus permitting colonization/infection with Pneumocystis [15,17]
Taken together the human studies clearly demonstrate evidence of a seasonal and/or climatological association and presentation with PCP, or serologic or autopsy detection of infection. A seasonal variation in incidence of PCP was first reported in 1991 [19]. Subsequently, the relationship between seasonality and temperature and hospitalization with PCP has described inconsistent findings with some, but not all studies describing any association. In both HIV-infected and uninfected patients the risk of PCP has been observed to be higher in summer (London, UK) [28,35], (Geneva, Switzerland [29] (Munich, Germany) [36] and (San Francisco, USA) [6] autumn (Melbourne, Australia) [41], and winter (Seville, Spain) [40]. As previously suggested [6], these differing results might be as a result of differences in patient populations, climatological factors, geography, Pneumocystis genotypes, or to differences in study design [6]. Because of geographical climatological differences, it is likely that summer temperatures in one country, or region, are similar to autumn or winter temperatures in another geographical location. For example, in Spain winters are generally mild (most regions having an average temperature between 10 and 20°C), and summers are hot (with average temperatures greater than 30°C) [40]. Thus, the average temperature in a Spanish winter is similar to that observed in other seasons in other countries during which the highest incidence of PCP has been described [40]. In London the “peak” summer temperature was 13°C [28], the mean summer temperature in San Francisco was 17.6°C [6], and average temperature in Melbourne was between 13.2 and 20°C [7,40]. Taken together, interpretation of these data, infer that rather than a seasonal association, presentation with PCP appears to be highest during the season of the year when the average temperature is between 10 and 20°C [40]. As previously noted [40], among HIV-infected persons, other confounding factors, including tobacco smoking [24], chronic obstructive pulmonary disease, bacterial pneumonia, and colonization of the respiratory tract by P. jirovecii, can contribute to increased risk of developing PCP, and also that these contributing factors additionally, are potentially affected by climatological factors [24].
A similar seasonal association is apparent in the two studies of infants done in Chile [25,26]. In the autopsy study the highest rate of detection of Pneumocystis was in winter (June-August), when average maximum temperatures were 16-18°C, and the lowest rate of detection was in autumn (March-May), when average maximum temperatures were 20-28°C [7,25]. In the study of serum antibody responses to Pneumocystis MsgA constructs among immune competent infants the lowest peaks were also detected in autumn (March-May) [26].
4.3 Seasonal Factors – Humidity and Precipitation
There is a less certain association between the climatological variables of humidity and precipitation, and detection of Pneumocystis in animal and human hosts, or with presentation with PCP in humans. Some animal studies [12,17,18] and some human studies [20,27,28,33] suggest an association, but others do not. Confounding these observations is the fact that data about this climatological variable was not routinely collected in either the animal or human studies.
A further potential factor, confounding interpretation of data concerning both temperature and humidity/precipitation in humans is that in the EuroSIDA study, which was a prospective observational cohort study that reported diagnosis of PCP in North, Central, and South Europe, the higher prevalence of PCP described in cooler, wetter North European climates might be explained by other logistical factors, including better access to healthcare (and so the likelihood of being diagnosed with PCP) in North Europe [30]. A similar interpretation can be applied to the findings from another European retrospective multicenter study [31].
4.4 Seasonal Factors – Outdoor Activities
The study from Atlanta, USA showed spending time outdoors gardening, camping or hiking in the six months prior to hospitalization with pneumonia was strongly associated with risk of PCP [21]. Additionally, studies from Cincinnati and San Francisco, USA showed clustering of PCP by Zip Codes [22,24]: the former study showing more cases in affluent areas with more green space [22], the latter reporting more cases in residential areas containing parks and small yards [24]. Taken together, these three studies infer that susceptible individuals are at greater risk of developing PCP if they have increased opportunities for being outdoors, and thus possibly for increased environmental exposure to Pneumocystis.
4.5 Genetic Factors
Observations from national (Netherlands) and single-site London (UK) cohorts infer that there might be an ethnic/genetically-determined predisposition to development of PCP [32,37]. Single point mutations (SNPs) in genes associated with innate immune function are increasingly recognized as significant factors dictating an individual’s susceptibility to infection [49,50,51]. Two reports describe an association between SNPs and development of PCP in HIV-infected individuals [52,53]. The FcγIIa receptor, which binds to immune complexes to facilitate uptake of microbes, is encoded by a gene with synonymous (functional) polymorphisms that affects binding affinity to IgG. Participants in the Multicenter AIDS Cohort Study (MACS) with the FcγRIIa RR genotype progressed to AIDS (i.e., CD4 count <200 cells/uL) faster than participants with RH or HH genotypes [52]. By contrast, participants with an FcγRIIa HH genotype progressed more quickly to AIDS (defined by development of PCP) than those with other genotypes [52]. While the underpinning mechanisms remain uncertain, these results raise intriguing questions about the antibodies that opsonize Pneumocystis and interact with FcγRIIa receptors. The chemokine receptor CXCR6 is a co-receptor that facilitates fusion of HIV to CD4 cells. The ligand for CXCR6 (CCXCL16) is highly expressed in the lungs. A SNP in codon 3 (CXCR6-E3K) is common in African Americans and rare in Caucasians. The AIDS Link to Intravenous Experience (ALIVE) study, a prospective cohort study of predominantly African American HIV-infected drug users, examined the relationship of CXCR6 SNPs and development of PCP [53]. Time to development of PCP was similar among the genotypes, but subjects homozygous or heterozygous for CXCR6-3E were more likely to die after PCP (and thus had a shorter survival time) than subjects homozygous for CXCR6-3K [53].
The current epidemiology of pediatric PCP in the USA demonstrates that it is less frequently observed in HIV-infected children. By contrast cases associated with hematologic malignancy and primary immunodeficiency have become more prominent, infants being the most commonly affected [54]. Well-described mutations in MHC class II (bare lymphocyte syndrome), recombination activating genes (RAG) -1 and -2, signal transducer and activator of transcription (STAT) -3, and IL-21R that can be modeled in genetically engineered mice and P. murina infection, provide evidence of genetic susceptibility to Pneumocystis infection [55]. Taken together these data suggest a gene-environment interaction in the disease.
4.6 Air Pollution
Among the general population it is increasingly evident that ambient air pollution contributes to the global burden of respiratory disease, including asthma, Chronic Obstructive Pulmonary Disease, and pneumonia [56,57,58,59,60,61,62,63,64,65]. The studies identified in this systematic review originating from Spain and San Francisco, USA clearly demonstrate an association between ambient air pollution factors and presentation of HIV-infected adults with PCP [6,40]. It is intriguing that in one study there was no association with PM10, NO2, CO, or ozone, and an association was only evident for SO2 [6]. In the other study NO2, PM10, CO, and ozone were associated with risk of hospitalization with PCP [40]. This apparent difference may in part be explained by the fact that San Francisco is one of the least polluted cities in the United States and so it is possible that levels of these pollutants were below thresholds that can trigger respiratory complications [6].
The mechanisms by which ambient air pollution increases susceptibility to pulmonary infection are not well defined. Controlled exposure studies of single pollutants that have used cells, animals and human subjects indicate that ambient air pollutants alter innate lung immunity at multiple levels, including altered muco-ciliary function, respiratory epithelial cell dysfunction, impaired alveolar macrophage phagocytosis, and dysfunction of surfactant protein A and D [Reviewed in 6]. However, the effects of ambient air pollution factors on humoral immunity and serologic responses to pulmonary infection, and the immune-toxic effects of real-life exposures to ambient air pollution factors are poorly understood. In one study from San Francisco [42] PM10 and NO2 were independently associated with suppressed IgM (but not IgG) responses to Pneumocystis Msg constructs. It was suggested that PM10 particles encountering bronchus associated lymphoid tissue, might impair antigen presenting cell function, resulting in decreased activation of the humoral immune system and suppressed serologic responses [42]. Both animal and human exposure studies have found mixed effects of NO2 inhalation on bronchoalveolar and systemic antibody responses [66,67,68,69]. In a second study also from San Francisco patients with PCP increasing exposure to ozone was associated with reduced BAL fluid IgA responses to P. jirovecii Msg constructs and increasing exposure to NO2 was independently associated with increased BAL fluid IgA responses to P. jirovecii Msg [43]. These results might be explained by the observation that ozone is a potent oxidant resulting in both bronchoalveolar, and systemic inflammation. Animal studies have found that rats in the first two weeks of exposure to ozone demonstrate decreased antibody responses to microbial antigens such as Listeria spp [70].
5. Conclusions
This systematic review found evidence of associations between Pneumocystis infection in both animal and human hosts, and climatological and environmental air pollution factors. These data are limited by inconsistent and incomplete sampling methodology in both animal and human studies. Data from human studies infer that rather than a seasonal association, presentation with PCP appears to be highest when the average temperature is between 10 and 20°C. A potential confounder is possible genetically-determined predisposition to, or protection from infection. There is evidence of an association with hospitalization with PCP and ambient air pollution factors, as well as a clear effect of air pollution on both systemic and bronchoscopic lavage fluid humoral responses to Pneumocystis. The results of this systematic review provide an improved understanding of the conditions involved in transmission of Pneumocystis in both animal and human hosts. Such knowledge is critical to efforts aimed at prevention of infection.
Acknowledgments
We thank Professor Olga Matos and Dr Christiana Weissenbacher-Lang, for helpful discussions about their publications. Both provided additional data that was not included in their original publications. Additionally, Professor Matos provided us with climatological data (temperature, humidity and precipitation) which was obtained from the Portuguese Weather Institute.
Author Contributions
Developed the concept for this article: PDW; performed the literature search: LH & RFM; wrote the manuscript: RFM; critically reviewed drafts of the manuscript for intellectual content, and approved the final submitted manuscript: PDW, LH, RFM.
Funding
PDW and RFM received no funding for this work. LH was funded by NIH K24 HL087713 and NIH R01 HL128156.
Competing Interests
The authors have declared that no competing interests exist.
References
- Miller RF, Smulian AG, Walzer PD. Pneumocystis species. Chapter 269 in: Mandell, Douglas, and Bennett’s Principles and Practise of Infectious Diseases: 9th Edition. Editors: GL Mandell, JE Bennett, and R Dolin. Elsevier Science. 2018 (in press). [Google scholar]
- Brown GD, Denning D, Gow NA, Levitz SM, Neta MG, White TG. Hidden killers: human fungal infections. Sci Transl Med. 2012; 4: 165rv13. [CrossRef] [Google scholar] [PubMed]
- Schildgen V, Mai S, Khalfaoui S, Lüsebrink J, Pieper M, Tillmann RL, et al. Pneumocystis jirovecii can be productively cultured in differentiated CuFi8 airway cells. MBio. 2014; 5: e01186-14.5. [Google scholar]
- Cushion MT, Linke MJ, Ashbaugh A, Sesterhenn T, C-ollins MS, Lynch K, et al. Echinocandin treatment of Pneumocystis pneumonia in rodent models depletes cysts leaving trophic burdens that cannot transmit the infection. PLoS One. 2010; 29: e8524. [CrossRef] [Google scholar] [PubMed]
- Yiannakis EP, Boswell TC. Systematic review of outbreaks of Pneumocystis jirovecii pneumonia: evidence that P. jirovecii is a transmissible organism and the implications for healthcare infection control. J Hosp Infect. 2016; 93: 1-8. [CrossRef] [Google scholar] [PubMed]
- Djawe K, Levin L, Swartzman A, Fong S. Roth B, Grieco K et al. Environmental risk factors for Pneumocystis pneumonia hospitalizations in HIV patients. Clin Infect Dis. 2013; 56: 74-81. [CrossRef] [Google scholar] [PubMed]
- World Weather Online: https://www.worldweatheroneline.com [Accessed 02 August 2018].
- Šebek Z, Rosický B. The finding of Pneumocystis carinii in shrews (Insectivora: Soricidae). Folia Parasitologica. 1967; 14: 263-267. [Google scholar]
- Poelma FG, Broekhuizen S. Pneumocystis carinii in hares, Lepus europaeus Pallas, in The Netherlands. Z Parasitenk. 1972; 40: 195-202. [Google scholar]
- Shiota T, Kurimoto H, Yoshida Y. Prevalence of Pneumocystis carinii in wild rodents in Japan. Zentralbl Bakteriol Mikrobiol Hyg A. 1986; 261: 381-389. [CrossRef] [Google scholar] [PubMed]
- Laakkonen J, Henttonen H, Niemimaa J, Soveri T. Seasonal dynamics of Pneumocystis carinii in field vole, Microtus agrestis, and common shrew, Sorex araneus, in Finland. Parasitol. 1999; 118: 1-5. [CrossRef] [Google scholar] [PubMed]
- Demanche C, Wanert F, Herrenschmidt N, Moussu C, Durand-Joly I, Dei-Cas E, et al. Influence of climatic factors on Pneumocystis carriage within a socially organized group of immunocompetent macaques (Macaca fascicularis). J Eukaryot Microbiol. 2003; 50: 611-613. [CrossRef] [Google scholar] [PubMed]
- Icenhour CR, Arnold J, Medvedovic M, Cushion MT. Competitive coexistence of two Pneumocystis species. Infect Genet Evol. 2005; 6: 177-186. [CrossRef] [Google scholar] [PubMed]
- Sanches EM, Pascador C, Rozza D, Ravazzolo AP, Driemiers D, Ravazzolo AP, et al. Detection of Pneumocystis spp. in lung samples from pigs in Brazil. Med Mycol. 2007; 45: 395-399. [CrossRef] [Google scholar] [PubMed]
- Kim KS, Jung JY, Kim JH, Kang S-C, Hwang E-K, Park B-K, et al. Epidemiological characteristics of pulmonary pneumocystosis and current infections in pigs in Jeju Island, Korea. J Vet Sci. 2011; 12: 15-19 [CrossRef] [Google scholar] [PubMed]
- Akbar H, Pinçon C, Aliouat-Denis C-M, Derouiche S, Taylor M-L, Pottier M, et al. Characterizing Pneumocystis in the lungs of bats: understanding Pneumocystis evolution and the spread of Pneumocystis organisms in mammal populations. Appl Environ Microbiol. 2012; 78: 8122–8136. [CrossRef] [Google scholar] [PubMed]
- Esgalhado R, Esteves F, Antunes F, Matos O. Study of the epidemiology of Pneumocystis carinii f. sp. suis in abattoir swine in Portugal. Med Mycol. 2013; 51: 66-71. [CrossRef] [Google scholar] [PubMed]
- Weissenbacher-Lang C, Kureljušić B, Nedorost N, Matula B, Schiessl W, Stienberger D, et al. Retrospective analysis of bacterial and viral co-infections in Pneumocystis spp. positive lung samples of Austrian pigs with pneumonia. PLoS One. 2016; 11: e0158479. [CrossRef] [Google scholar] [PubMed]
- Hoover DR, Graham NM, Bacellar H, Schrager LK, Kaslow R, Visscher B, et al. Epidemiologic patterns of upper respiratory illness and Pneumocystis carinii pneumonia in homosexual men. Am Rev Respir Dis. 1991; 44: 756-759. [CrossRef] [Google scholar] [PubMed]
- Baccetti P. Seasonal and other influences on United States AIDS incidence. Stat Med. 1994; 13: 1921-1931. [CrossRef] [Google scholar] [PubMed]
- Navin TR, Rimland D, Lennox JL, Jernigan J, Cetron M, Hightower A, et al. Risk factors for community-acquired pneumonia among persons infected with the human immunodeficiency virus. J Infect Dis. 2000: 181: 158-164. [CrossRef] [Google scholar] [PubMed]
- Dohn MN, White ML, Vigdorth EM, Buncher CR, Hertzberg VS, Baughman RP, et al. Geographic clustering of Pneumocystis carinii in patients with HIV infection. Am J Resp Crit Care Med. 2000; 162: 1617-1621. [CrossRef] [Google scholar] [PubMed]
- Morris AM, Swanson M, Ha H, Huang L. Geographic distribution of human immunodeficiency virus-associated Pneumocystis carinii pneumonia in San Francisco. Am J Respir Crit Care Med. 2000; 162: 1622-1626. [CrossRef] [Google scholar] [PubMed]
- Morris A. Kingsley LA, Groner G, Lebedeva IP, Beard CB, Norris KA. Prevalence and clinical predictors of Pneumocystis colonization among HIV-infected men. AIDS. 2004; 18: 793-798. [CrossRef] [Google scholar] [PubMed]
- Vargas SL, Ponce CA, Luchsinger V, Silva C, Gallo M, Lopez R, et al. Detection of Pneumocystis carinii f. sp. hominis and viruses in presumably immunocompetent infants who died in the hospital or in the community. J Infect Dis. 2005; 191: 122-126. [CrossRef] [Google scholar] [PubMed]
- Djawe K, Daly KR, Vargas SL, Santolaya ME, Ponce CA, Bustamante RM, et al. Seroepidemiological study of Pneumocystis jirovecii infection in healthy infants in Chile using recombinant fragments of the P. jirovecii major surface glycoprotein. Int J Infect Dis. 2010: 14: e1060-1066. [CrossRef] [Google scholar] [PubMed]
- Settnes OP, Genner J. Pneumocystis carinii in human lungs at autopsy. Scand J Infect Dis. 1986; 18: 489-496. [CrossRef] [Google scholar] [PubMed]
- Miller RF, Grant AD, Foley NM. Pneumocystis carinii pneumonia. Lancet. 1992; 339: 747-748. [CrossRef] [Google scholar] [PubMed]
- Vanhems P, Hirschel B, Morabia A. Seasonal incidence of Pneumocystis carinii pneumonia. Lancet. 1992; 339: 1182. [CrossRef] [Google scholar] [PubMed]
- Lundgren JD, Barton SE, Lazzarin A, Danner S, Goebel FD, Pehrson P, et al. Factors associated with the development of Pneumocystis carinii pneumonia in 5,025 European patients with AIDS. Clin Infect Dis. 1995; 21: 106-113. [CrossRef] [Google scholar] [PubMed]
- Delmas M-C, Schwoebel V, Heisterkamp SH, Downs AM, Ancelle-Park RA, Brunet JB, et al. Recent trends in Pneumocystis carinii pneumonia as AIDS-defining disease in nine European countries. J Acquir Immune Defic Syndr. 1995; 9: 74-80. [Google scholar]
- Del Amo J, Petruckevitch A, Phillips AN, Johnson AM, Stephenson J, Desmond N, et al. Spectrum of disease in Africans with AIDS in London. AIDS. 1996; 10: 1563-1569. [CrossRef] [Google scholar] [PubMed]
- Lubis N, Baylis D, Short A, Stebbing J, Teague A, Portsomuth S, et al. Prospective cohort study showing changes in the monthly incidence of Pneumocystis carinii pneumonia. Postgrad Med J. 2003; 79: 164-166. [CrossRef] [Google scholar] [PubMed]
- Miller RF, Evans HE, Copas AJ, Cassell JA. Climate and genotypes of Pneumocystis jirovecii. Clin Microbiol Infect. 2007; 13: 445-448. [CrossRef] [Google scholar] [PubMed]
- Walzer PD, Evans HER, Copas AJ, Edwards SG, Grant AD, Miller RF. Early predictors of mortality from Pneumocystis jirovecii pneumonia in HIV-infected patients. Clin Infect Dis. 2008; 46: 625-633. [CrossRef] [Google scholar] [PubMed]
- Sing A, Schmoldt S, Laubender RP, Heesemann J, Sing D, Wildner M. Seasonal variation of Pneumocystis jirovecii infection: analysis of underlying climactic factors. Clin Microbiol Infect. 2009; 15: 957-960. [CrossRef] [Google scholar] [PubMed]
- Schoffelen AF, van Lelyveld SF, Barth RE, Gras L, de Wolf F, Netea MG, et al; ATHENA national observational cohort study Lower incidence of Pneumocystis jirovecii pneumonia among Africans in the Netherlands host or environmental factors? AIDS. 2013, 27: 1179-1184. [CrossRef] [Google scholar] [PubMed]
- Varela JM, Regordán C, Medrano FJ, Respaldiza N, de la Hora C, Montes-Cano MA, et al. Climatic factors and Pneumocystis jirovecii infection in southern Spain. Clin Microbiol Infect. 2004; 10: 770-772. [CrossRef] [Google scholar] [PubMed]
- Calderón EJ, Varela JM, Medrano FJ, Nieto V, Gonzalez-Becarra C, Respaldiza N, et al. Epidemiology of Pneumocystis carinii pneumonia in southern Spain. Clin Microbiol Infect. 2004; 10: 673-676. [CrossRef] [Google scholar] [PubMed]
- Alvaro-Meca A, Palomares-Sancho I, Diaz A, Resino R, De Miguel AG, Resino S. Pneumocystis pneumonia in HIV-positive patients in Spain: epidemiology and environmental risk factors. J Int AIDS Soc. 2015; 18: 19906. [CrossRef] [Google scholar] [PubMed]
- Tadros S, Teichtahl AJ, Cicirello S, Wicks IP. Pneumocystis jirovecii pneumonia in systemic autoimmune rheumatic disease: a case–control study. Semin Arthritis Rheum. 2017; 46: 804–809. [CrossRef] [Google scholar] [PubMed]
- Blount RJ, Djawe K, Daly KR, Jarlsberg LG, Fong S, Balmes J, et al. Ambient air pollution associated with suppressed serologic responses to Pneumocystis jirovecii in a prospective cohort of HIV-infected patients with Pneumocystis pneumonia. PLoS One. 2013; 8: e80795. [CrossRef] [Google scholar] [PubMed]
- Blount RJ, Daly KR, Fong S, Chang E, Greico K, Greene M, et al. Effects of clinical and environmental factors on bronchoalveolar antibody responses to Pneumocystis jirovecii: a prospective cohort study of HIV+ patients. PLoS One. 2017; 12: e0180212. [CrossRef] [Google scholar] [PubMed]
- Bush RK. Aerobiology of pollen and fungal allergens. J Allergy Clin Immunol 1989; 84: 1120–44. [CrossRef] [Google scholar] [PubMed]
- Mezzari A, Perin C, Santos SA, Bernd LA. Airborne fungi in the city of Porto Alegre, Rio Grande do Sul, Brazil. Rev Inst Med Trop Sao Paulo. 2002; 44: 269–272. [CrossRef] [Google scholar] [PubMed]
- Menzes EA, Trindade EC, Costa MM, Freire CC, Calvalcante MdeS, Cunha FA. Airborne fungi isolated from Fortaleza city, state of Ceará, Brazil. Rev Inst Med Top S Paulo. 2004; 46: 133-137. [CrossRef] [Google scholar] [PubMed]
- Favero-Longo SE, Sandrone S, Matteucci E, Appolonia L, Piervittori R. Spores of lichen-forming fungi in the mycoaerosol and their relationships with climate factors. Sci Total Environ. 2014; 466-7: 26-33. [CrossRef] [Google scholar] [PubMed]
- Grinn-Gofron A, Strzelczak A, Przestrzelska K. Seasonal variation of Ganoderma spore concentrations in urban and suburban districts of the city of Szczecin, Poland. Ann Agric Environ Med. 2015; 22: 6-10. [CrossRef] [Google scholar] [PubMed]
- Lupiañez CB, Canet LM, Carvalho A, Alcazar-Fuoli L, Springer J, Lackner M, et al. Polymorphisms in host immunity-modulating genes and risk of invasive aspergillosis: results from the AspBIOmics consortium. Infect Immun. 2015; 84: 643-657. [CrossRef] [Google scholar] [PubMed]
- Salas A, Pardo-Seco J, Barral-Arca R, Cebey-Lopez M, Gomez-Carballa A, Rivero-Calle I, et al; GENDRES Network. Whole exome sequencing identifies new host genomic susceptibility factors in empyema caused by Streptococcus pneumoniae in children: a pilot study. Genes (Basel). 2018; 9. pii: E240. [CrossRef] [Google scholar] [PubMed]
- Kondoh T, Letko M, Munster VJ, Manzoor R, Maruyama J, Furuyama W, et al. Single nucleotide polymorphisms in human NPC1 influence filovirus entry into cells. J Infect Dis. 2018 Jul 14. [Epub ahead of print] [CrossRef] [Google scholar] [PubMed]
- Forthal DN, Landucci G, Bream J, Jacobson LP, Phan TB, Montoya B. FcγRIIa genotype predicts progression of HIV infection. J Immunol. 2007; 179: 7916–7923. [CrossRef] [Google scholar] [PubMed]
- Duggal P, An P, Beaty TH, Strathdee SA, Farzadagen H, Markham RB, et al. Genetic influence of CXCR6 chemokine receptor alleles on PCP-mediated AIDS progression among African Americans. Gen Immun. 2003; 4: 245-250. [CrossRef] [Google scholar] [PubMed]
- Inagaki K, Blackshear C, Hobbs CV. Pneumocystis infection in children: National trends and characteristics in the United States, 1997-2012. Pediatr Infect Dis J. 2018 May 21. [Epub ahead of print]. [CrossRef] [Google scholar] [PubMed]
- Elsegeiny W, Zheng M, Eddens T, Gallo RL, Dai G, Trevejo-Nunez G, et al. Murine models of Pneumocystis infection recapitulate human primary immune disorders. JCI Insight. 2018; 3: e91894. [CrossRef] [Google scholar] [PubMed]
- Lim SS, Vos T, Flaxman AD, Danael G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012; 380: 2224-2260. [CrossRef] [Google scholar] [PubMed]
- Laumbach RJ, Kipen HM. Respiratory health effects of air pollution: update on biomass smoke and traffic pollution. J Allergy Clin Immunol. 2012; 129: 3-11. [CrossRef] [Google scholar] [PubMed]
- Xiao Q, Liu Y, Mulholland JA, Russell AG, Darrow LA, Tolbert PE, et al. Pediatric emergency department visits and ambient air pollution in the US State of Georgia: a case-crossover study. Environ Health. 2016; 15: 115. [CrossRef] [Google scholar] [PubMed]
- Neupane B, Jerrett M, Burnett RT, Marrie T, Arain A, Loeb M. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am J Respir Crit Care Med. 2010; 181: 47–53. [CrossRef] [Google scholar] [PubMed]
- Cheng M-H, Chiu H-F, Yang C-Y. Coarse particulate air pollution associated with increased risk of hospital admissions for respiratory diseases in a tropical city, Kaohsiung, Taiwan. Int J Environ Res Public Health. 2015; 12: 13053-13068. [CrossRef] [Google scholar] [PubMed]
- Pirozzi CS, Jones BE, VanDerslice JA, Zhang Y, Paine R, Dean NC. Short-term air pollution and incident pneumonia. A case-crossover study. Ann Am Thorac Soc. 2018; 15: 449-459. [CrossRef] [Google scholar] [PubMed]
- Li D, Wang JB, Zhang ZY, Shen P, Zheng PW, Jin MJ, et al. Effects of air pollution on hospital visits for pneumonia in children: a two-year analysis from China. Environ Sci Pollut Res Int. 2018; 25: 10049-10057. [CrossRef] [Google scholar] [PubMed]
- Medina-Ramon M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol. 2006; 163: 579–588. [CrossRef] [Google scholar] [PubMed]
- Kim CS, Alexis NE, Rappold AG, Kehrl H, Hazucha MJ, Lay JC, et al. Lung function and inflammatory responses in healthy young adults exposed to 0.06 ppm ozone for 6.6 hours. Am J Respir Crit Care Med. 2011; 183: 1215-1221. [CrossRef] [Google scholar] [PubMed]
- Arjomandi M, Wong H, Donde A, Frelinger J, Dalton S, Ching W, et al. Exposure to medium and high ambient levels of ozone causes adverse systemic inflammatory and cardiac autonomic effects. Am J Physiol Heart Circ Physiol. 2015; 308: 1499-1509. [CrossRef] [Google scholar] [PubMed]
- Ehrlich R, Silverstein E, Maigetter R, Fenters JD. Immunologic response in vaccinated mice during long-term exposure to nitrogen dioxide. Environ Res. 1975; 10: 217-223. [CrossRef] [Google scholar] [PubMed]
- Fujimaki H, Shimizu F, Kubota K. Suppression of antibody response in mice by acute exposure to nitrogen dioxide: in vitro study. Environ Res. 1981; 26: 490-496. [CrossRef] [Google scholar] [PubMed]
- Balchum OJ, Buckley RD, Sherwin R, Gardner M. Nitrogen dioxide inhalation and lung antibodies. Arch Environ Health. 1965; 10: 274-277. [CrossRef] [Google scholar] [PubMed]
- Hidekazu F, Fujio S. Effects of acute exposure to nitrogen dioxide on primary antibody response. Arch Environ Health. 1981; 36: 114-119. [CrossRef] [Google scholar] [PubMed]
- Jakab GJ, Spannhake EW, Canning BJ, Kleeberger SR, Gilmour MI. The effects of ozone on immune function. Environ Health Perspect. 1995; 103 Suppl 2: 77-89. [Google scholar]



