Improving the HealthCare of People with Dementia beyond the Diagnosis: The “Carlo Poma Dementia Care Pathway” Study Protocol
Vincenza Frisardi 1, * ![]() , Sara Faroni 2
, Sara Faroni 2 ![]() , Angela Bellani 2
, Angela Bellani 2 ![]() , Felice Biagi 1
, Felice Biagi 1 ![]() , Emanuela Galante 1
, Emanuela Galante 1 ![]() , Alida Balzanelli 1
, Alida Balzanelli 1 ![]() , Erika Talassi 1
, Erika Talassi 1 ![]() , Lorella Frittoli 1
, Lorella Frittoli 1 ![]() , Giancarla Capiluppi 2
, Giancarla Capiluppi 2 ![]() , Claudia D’angelis 1
, Claudia D’angelis 1 ![]() , Antonella Taragnani 2
, Antonella Taragnani 2 ![]() , Donatella Terzi 2
, Donatella Terzi 2 ![]() , Elena Podavini 2
, Elena Podavini 2 ![]() , Graziana Gazzoni 3
, Graziana Gazzoni 3 ![]() , Graziana Simoncelli 3
, Graziana Simoncelli 3 ![]() , Carmine Matarazzo 4
, Carmine Matarazzo 4 ![]() , Mirko Avesani 1
, Mirko Avesani 1 ![]() , Antonio Ventura 5
, Antonio Ventura 5 ![]() , Consuelo Basili 6
, Consuelo Basili 6 ![]() , Maurizio Galavotti 6
, Maurizio Galavotti 6 ![]() , Carlo Maria Stucchi 1
, Carlo Maria Stucchi 1 ![]() , Maria Cristina Cilia 1
, Maria Cristina Cilia 1 ![]() , Alfonso Ciccone 1
, Alfonso Ciccone 1 ![]()
- Neurological Department, Asst Mantova, Carlo Poma Hospital, Mantua, Italy
- Frailty Department, Multiservice center, Asst Mantova Carlo Poma Hospital, Mantua, Italy
- Frailty Department, Social Workers Office-Asst Mantova Carlo Poma Hospital, Mantua, Italy
- Rehabilitation Department Asst Mantova, Bozzolo, Italy
- Statistical Department Asst Mantova, Carlo Poma Hospital, Mantua, Italy
- Strategical Direction Department, Asst Mantova Carlo Poma Hospital, Mantua, Italy
* Correspondence: Vincenza Frisardi ![]()
Academic Editor: Michael Fossel
Received: October 31, 2018 | Accepted: February 13, 2019 | Published: February 27, 2019
OBM Geriatrics 2019, Volume 3, Issue 1 doi:10.21926/obm.geriatr.1901036
Recommended citation: Frisardi V, Faroni S, Bellani A, Biagi F, Galante E, Balzanelli A, Talassi E, Frittoli L, Capiluppi G, D’angelis C,Taragnani A, Terzi D, Podavini E, Gazzoni G, Simoncelli G, Matarazzo C, Avesani M, Ventura A, Basili C, Galavotti M, Stucchi CM, Cilia MC, Ciccone A. Improving the HealthCare of People with Dementia beyond the Diagnosis: The “Carlo Poma Dementia Care Pathway” Study Protocol. OBM Geriatrics 2019; 3(1): 036; doi:10.21926/obm.geriatr.1901036
© 2019 by the authors. This is an open access article distributed under the conditions of the Creative Commons by Attribution License, which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is correctly cited.
Abstract
The WHO global action plan on the public health response to dementia 2017-2025 stressed the need to have a comprehensive approach with deep interconnections and cross-cutting elements through several action areas. As the elderly population grows worldwide, the number of patients with dementia increases rapidly because age is an important risk factor for developing late-onset dementia. Currently, dementia syndrome represents a true emergency. Once a diagnosis of dementia was made, informal caregivers, patients and their household, they swing between desires, fear, concerns about the present and the future. In fact, in several clinical context there is not a possibility to have specific services able to address the critical issues, which could happen along the disease’s course. Despite extensive research in the field of dementia, there still exist a deficiency in the quality of dementia care. There is a paucity of robust results concerning the care experiences of patients with dementia. It is mandatory to understand these experiences if we want to address care inequalities and create impactful dementia policies to improve services for supporting individuals and family caring, and promoting a good quality of life for all people affected by this devastating disease. Aim of this article is to describe the development of an operational protocol to improve the healthcare of people with cognitive impairment and their family. Unfortunately, it is not always easy to assure the whole services that a patient with dementia (PwD) needs. Frequently, this is due to a lack of communication between public hospitals and local authorities. We believe that it is possible to improve the quality of life of PwD and to optimize the public health expenditure through the creation of a specific care network. The services integration means to guarantee the continuity of care and the appropriateness of access to health care, avoiding inappropriate use. This could result in both reducing the healthcare costs and saving resources.
Graphical abstract

Keywords
Dementia care pathway; acute setting; organizational model
1. Background
Dementia is an umbrella term for several progressive neurodegenerative diseases. Sometimes, the first clinical manifestation is the occurrence of behavioral and psychological or motor disorders; thus, dementia is often underdiagnosed and mistreated for a long period of time [1]. Alzheimer’s disease (AD) is the most common form of dementia characterized by memory loss and impairment in cognitive and behavior abilities. It interferes significantly with the functional independence and social relationship [2] of people with dementia (PwD). The prevalence of Alzheimer's disease in Europe was estimated at 5.05% (men 3.31%; women, 7.13%) while the incidence was 11.08 per 1000 person-years (7.02 per 1000 person-years in men and 13.25 in female per 1000 person-years in women) [3]. This phenomenon is expected to increase worldwide [4]. Recent update about age specific data from East Asia and Africa predicted an increase of 15% and 1%, respectively, by 2030 and 2050 on the overall world estimates, suggesting a very global explosion of this disease [2]. The World Health Organization (WHO) global action plan on the public health response to dementia 2017-2025 stressed the need to have a comprehensive and multisector approach with deep interconnections and cross-cutting elements through several action areas, with emphasis on promotion, prevention, treatment, rehabilitation and care [5]. This plan against dementia envisions “a world in which people can live well with or without dementia, and receive the supports they need to fulfill their potential with dignity, respect and equality” [5]. Furthermore, one of the major issue is the “balance between cure and care for PwD and their caregivers” utilizing knowledge, best practice and experience to improve dementia prevention, risk reduction, care and support. Additionally, it is necessary to generate new knowledge towards finding disease-modifying treatments, as well as effective risk reduction's interventions and innovative models of care [5]. Dementia is one of major causes of disability and dependency among the elderly [6]. It is associated with the worst health outcomes [6]. Very often, clinicians delay the diagnosis of heart or pulmonary illness in these patients because PwD are unable to explain their symptoms appropriately [7]. Dementia is not only a problem of forgetting. Behavioral and psychological disorders (BPSDs) occur in most patients [8]. BPSDs can cause immense patient suffering and are responsible for both their hospitalization or institutionalization and caregiver distress [9,10]. Identification of predisposing and precipitating factors is very important. High rates of emergency department attendance could indicate inadequate availability of care in the community, a paucity of advance directives, or lack of focus on patients' quality of life rather than to an acute illness [11]. In fact, the emergency department becomes the only “escape” for unbearable situations at home or in nursing home [10,11]. While a good quality of life is the aim in caring for PwD, effective methods for evaluating this outcome are lacking. Moreover, dementia leads to the increased long-term costs for governments, communities, families and individuals, and loss in productivity for economies [4]. In 2015, dementia costs were estimated at US818 billion; by 2030, this is expected to reach the US2 trillion, a total that could undermine social and economic development and overwhelm health and social care systems [4,5]. Coping with this emergency, it is important to have available a qualified and integrated network home care and health services. In theory, the global dementia plan describes an ambitious perspective for a timely identification of this disease while providing the interventions to integrate for supporting dementia dyad (PwD and their caregiver) [5]. The notion of critical or integrated care pathway (ICP) has been used in different health settings [12,13,14]. The European Pathway Association defines a care pathway as “a complex of intervention for the mutual decision-making and organization of care process for a well-defined group of patients during a well-defined period” [12]. It is important to have an outline of anticipated care for dementia given that its trajectory is variable but ineluctable. It is essential to have a multidisciplinary team in an appropriate framework with the aim to support PwD and their family through the clinical experience. An uncertain illness trajectory and the unpredictable levels of cognitive deterioration, both of them typify dementia’s syndrome; in this context, a dementia care pathway may be attractive. The dementia care pathway developed in England by the National Institute of Health and Clinical Excellence (NICE) has its starting point in the staff training. These guidelines represent a holistic support for practitioners [15]. The NICE pathway emphasizes the post-diagnostic phase promoting choices that support caregiver at home and maintaining as longer as possible the patient’s independence [15]. Commonly, these pathways were built upon the scientific evidence. Nevertheless, they do not enjoy worldwide distribution because the ideal path for treating dementia often has to deal with local bureaucratic problems. The term pathway could suggest a roadmap where patients and their caregivers are placed on and where clinicians and health providers could have the total control. In our opinion, we consider it rather as a “net” made up by clinical, social and political providers (Figure 1). The purpose of this ICP is, firstly, to diagnose cognitive impairment promptly; secondly, to intercept the future needs that every patient could face. Although these aspects are shared and well accepted both by politicians and care providers, at the best of our knowledge, there are not some robust evidence in the operationalization of this revolutionary mission for dementia healthcare beyond the diagnosis moment. A lack of evidence about the ICP experiences means that we do not know how their idealized version could be managed in real practice. We need to determine the care trajectory that could improve the quality of life of these patients, and this is the priority of any political intervention.
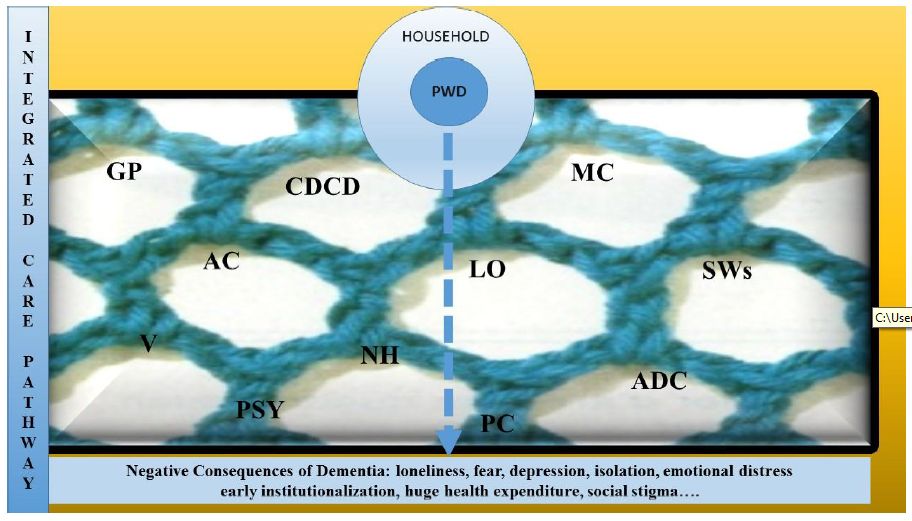
Figure 1 PWD, People With Dementia, GP, General Practitioner, CDCD, Center For Diagnosis Of Cognitive Disorders, MC, Multiservice Center, LO, Legal Office, SWs, Social Workers, V, Volunteers, PSY, Psychologists, AC, Alzheimer’s Café, NH, Nursing Home, PC, Palliative Care, ADC, Adult Day Care.
In this article, we describe the operational protocol about the Clinical Pathway for PwD (named the Diagnostic-Therapeutic & Healthcare Pathway for PwD specifically). It has been set up in the “Carlo Poma” Hospital, in Mantua, a city in the Northeast of Italy. Therefore, the purpose of this article is to present how we have planned the support network for PwD and their family in order to meet the National [16] and International [5] Guidelines for dementia.
In fact, the principal goals of this project are:
-
1) Facilitating the early diagnosis of cognitive disorder
-
2) Reducing the waiting time before to perform preliminary and specific tests
-
3) Reducing the waiting time between the first visit and the formulation of diagnosis and starting therapy
-
4) Ensuring a good quality of life, preventing complications disease-related including family distress
-
5) Delaying institutionalization
-
6) Decreasing the access to the emergency department for BPSD management
-
7) Reducing the hospitalization rate
-
8) Creating a global service (point of reference for patients, their families, their caregivers, general practitioners (GPs) and other colleagues in acute departments where sometime PwD are admitted for other medical/surgical conditions).
2. Materials and Methods
A literature review was performed by searching the following terms “dementia care”, “dementia plan”, “dementia network”, “dementia strategies”, “dementia caring” on PubMed, Ovid, and ResearchGate. In addition, articles from pertinent sources, such as Italian government’s reports and related website especially for local health legislature, were included in our search. We reported our project according to the SQUIRE Guidelines V.2.0, (Standard for Quality Improvement Reporting Excellence) [17]. A panel of 11 experts (neurologists, social workers, radiologists, neuropsychologist and statisticians of “Carlo Poma Hospital”) has planned this protocol. For validation of preliminary draft, a modified Delphi technique was employed [18]. The responsible of Quality Management Office of our hospital verified the final draft that finally was approved by the Head Health Manager. This protocol was implemented in our Center for Diagnosis of Cognitive Disorders (CDCD) at the Neurological Department of our hospital, with data collection starting from 1 July 2017. Furthermore, data will be analyzed, and findings will be reported after a period of at least two years. An improvement group (with representatives of the entire professional area involved in this project) was set up to address the upcoming critical aspects during the application of the protocol.
After four Delphi rounds, five topical moments in this pathway have reached a consensus (Table 1):
1. Early symptoms identification and first service encounters
2. Assessment process
3. Diagnostic disclosure and Integration of Treatment’s Plan
4. Follow-ups
5. Post-diagnostic support and appropriate interventions until the end-life stage.
We have enclosed in this protocol several care professional: experts who work in the CDCD (neurologists, geriatricians and neuropsychologists), a trained nurse who represents the case officer of CDCD, social workers, and legal representatives. The CDCD staff works closely with the Multiservice Center (MC). MC is a multidisciplinary service that help people living with precarious conditions (including PwD) to resolve social, financial and administrative problems. In fact, thanks to the cooperation among nurses, physical therapists and protective services from municipal district, the MC has the possibility to activate readily helpful services (for example the Adult Day center, nursing home, residential care) and other social measure to support any difficulties that every patients and their family could have during the disease’s course.
Table 1 Integrated care pathway for people with dementia and their families: Principal phases.

Three psychologists with a proved experience in dementia field will administer standardized and normed psychological tests. Based on compendia of cognitive testing [19,20] and the recommendations from the Italian Neuropsychological Society [21], the psychologists will perform a comprehensive cognitive assessment-Italian version for all enrolled patients (Table 2). Furthermore, the functional status and the social and family load of dementia will be evaluated too (Table 2).
Table 2 Global assessment for people with dementia.

We have strengthened the role of general practitioners (GPs) as the first promoters of an initial assessment as well as of an early refer to the specialist consultant for any suspected case. For this purpose, we have organized some informative meetings to explain our project to all of our urban and non-urban districts GPs, In our project, the GPs have to perform the General Practitioner Cognitive Assessment (GP-Cog) test-Italian version [39] in persons with suspected cognitive impairment and in persons at increased risk of cognitive impairment. GP-Cog takes no longer than five minutes to administer this test that comprises two components: a six item cognitive assessment with the patient and an informant questionnaire if the cognitive assessment score is equivocal (between 5-8). Scores > 8 are deemed to represent cognitive impairment and < 5 intact cognition (sensitivity 82-85%; specificity 83-86) [40]. GPs may contact the CDCD specialist both directly by an email or phone and indirectly by a communication to the MC. A nurse is always available to receive whichever questions or concerns from the users (GPs, patients, caregivers). We have also planned a monitoring chart to detect each variation of clinical status of patient and the improvement or not of caregiver distress during the period between the follow-ups. For this purpose, a nurse from MC was assigned to complete this monitoring chart by a phone interview to caregiver, especially when the specialist reports some situation at risk (distressful behavior, changes in therapy, delirium, high distress of caregiver and so on). Our project is oriented to manage both clinical and social condition of patients. In this respect, we formalized the principal situations that make necessary a close connection (by phone or email) between specialists, GPs and MC in order to counteract promptly all the emerging situations that alters the frail balance of our users (Table 3).
A trained nurse from the CDCD works closely with one nurse from the MC. They represent the interface between patients’ needs and the clinical and social providers. In order to facilitate the access to dementia care pathway for our patients without any concerns from the caregiver, they book the appointments personally.
Table 3. Precipitating factors that make necessary a close connection among the ICP principal clinical providers (General Practitioner, Specialist, Multiservice Center).

2.1 The Screening Moment
With a GP-Cog score pathological or borderline, GPs have to send a communication to the MC. Sometime people contact directly the private specialists for a visit. In addition, other specialists may contact the MC indicating the name of patient who could benefit for a second level of intervention. GPs, specialists from other hospitals and citizens may consult a webpage site of our hospital to contact the staff [41]. Alternatively, after a first neurological evaluation or hospitalization for other causes, people at high suspicious of cognitive impairment are addressed to the CDCD (for example, patients who developed delirium, or showed clear sign of cognitive impairment but without a previous diagnosis of dementia) [42,43].
2.2 The Referral Moment
This is the step where patients and their family are enrolled in this ICP. Initially, the case officer of CDCD (she is a trained nurse who works in the Neurological Department) schedules the clinical visit for patient and their family. She cope with every problem that PwD and their family may present into access to ICP. Patients have to perform all the planned investigations depending on the clinical suspicion in according to the International Guidelines on Dementia (blood tests, instrumental examinations, and neuropsychological assessment); subsequently, the case officer makes an appointment directly avoiding further passages for caregivers in other offices. In Italy, in fact, a center for reservation of medical appointments fixes the date depending on the free place on agenda without considering the case priority.
2.3 The Diagnostic Moment
In addition to the basal investigations, our neuropsychologists perform a comprehensive neuropsychological assessment. The neuropsychologists produce a comprehensive report (a detailed history and two to three hours of paper-and-pencil tests) for the family and the CDCD ' s specialist, offering specific recommendations for further treatment or timing for re-evaluation. After this, the nurse from MC contact patients for the booked specialist consultation (neurologist or geriatrician with a proven experience in the field of dementia) who decides if there are need to other investigations or it is possible to start with therapy. At this point, the social workers in our group intervene to assist the caregivers in order to plan useful measures able to support them (open home, respite care, voucher for municipal assistance). Moreover, they explain the legal consequence of this disease.
2.4 The Follow-Ups
We have standardized the timing of follow-ups (respectively at 1, 3 and 6 month after the diagnostic moment). These appointments are useful for clinicians to check the pharmacological effects and to monitor the clinical condition and the variation about the abilities to live independently. Moreover, they can also intercept every problem in disease management at home. In fact, at the end of each follow-up, an e-mail summarizing the final report is sent from the case officer of CDCD to the specialist consultant and the MC contemporaneously. This communication allows us to know the updated situation promptly.
2.5 Post-Diagnostic Moment
After the first six months, clinicians and neuropsychologists re-evaluate the patients’ history and disease course. In this way, it is possible to organize specific strategies and programs. Sometime, we need to launch pro-active policies to support the vulnerability of the whole household. Actually, this last phase depends a lot on the disease’s stage. In fact, if a patient starts its pathway when he/she is in the middle or late stage of dementia, any social, protective and pro-active measures have to be realized as soon as possible, often at the beginning of ICP or concurrent with the clinical appointments.
2.6 Caregivers’ Support
During the appointment with the specialist, the MC staff give instructions to the caregiver about some measures that could be implemented at home. Likewise, they seek to increase the awareness of the necessity to activate a legal protection, drawing up a social and caring profile. MC staff also perform a triage schedule that includes the assessment scale for social frailty and caregiver distress (Table 1). In particular, the seven-item social frailty index and the Relative Stress Scale (RSS). The first assesses some items like living arrangements, education, socioeconomic status, social network, and support providing a score between “0” and “7”, with higher scores indicating higher levels of social frailty.
RSS covers emotional distress, restrictions on the caregivers’ social life and negative feelings directed towards the patient. It consists of 15 items and each item is scored at five levels of intensity, from zero = 'not at all' to four = 'to a high degree'. Moreover, we also evaluated the caregiver distress by the Distress Scale of the Neuropsychiatric inventory (NPI-D). NPI-D measures the distress associated with the patient’s BPSD. This test covers the 12 items of the NPI and is rated on a six-level scale ranging from 0 (“not at all”) to five (“very severely or extremely”). The higher the sum score, the higher is the caregiver’s distress. Finally, MC completes the personalized care plan identifying the case-care manager trained to monitor the situation of our patients at home.
Alongside this pathway, psychological support groups were established for caregivers. Aim of these groups were to reduce the negative impact once a diagnosis of dementia was made. These groups not only provides support but also helps to build the resilience process. Not only do they provide education about how to self-manage their loved one at home but also about financial support whom they can benefit from regional or governmental measures.
2.7 Monitoring System
A computer technology company has implemented some software for our hospital. This software allows reporting when PwD is admitted to emergency room or in other medical or surgical department (the name of this program is Healthcare Portal that enters in the Electronic Informative Healthcare System of our region) [44]. In fact, when PwD is admitted into the emergency department for other medical conditions, the electronic triage sheet provides a symbol to identify people belonging to ICP and they are reported quickly to the MC. This latter, then, will inform the CDCD’s specialist in order to evaluate the patient during the hospitalization, avoiding the delirium onset or other complications where it is possible. Furthermore, MC activates the procedures for a prompt discharge at home or in other community services with the necessary devices (i.e., wheelchairs).
To report social needs of these patients (especially in the hospital discharge moment), we use a computerized notification form (the MAntua Intranet Ats, MAIA system) [14] so that the professional figures working in the primary care may know in real time the needs for PwD at home.
The “Carlo Poma” ethics committee approved this project; then, a referent belonging to the Research Quality Association verified the protocol formulation. The code of this protocol is PDTA 70.
3. Discussion
Dementia is a de-structuring disease that affects the individual abilities to live independently and to relate itself with other people. Our “Diagnostic-Therapeutic & Healthcare Pathway for PwD” is an operative integrated care protocol that was born in order to create a multi-professional network able to support PwD and their families. This ICP has been developed inside of an acute setting, that is, in the principal hospital of our district (the Carlo Poma Hospital, in Mantua, Lombardy, Italy). In this hospital, a dedicated staff coordinates multiple services coping with any emerging needs of our patients. In this way, we wanted to help PwD and their families to survive to the terrible consequences of this disease including its negative impact on social life (Figure 1). The Carlo Poma Hospital has both a mission and vision. The mission is to compete with all the other stakeholders on the provision of essential assistance levels, in order to protect and promote physical and mental health. Furthermore, it works to ensure a safe life for all of the elderly living with dementia in their life context, through a model of integrated assistance between hospital and community. Furthermore, it works to ensure a safe life for all of the elderly living with dementia in their life context, through a model of integrated assistance between hospital and community. The vision of our company is "from treatment to take care", especially of chronic and fragile patients and their families. The promotion of participation and valorization of volunteering is encouraged too. In line with the international literature, our hospital recognizes the importance to have a family and community support for PwD [46,47]. For this reason, a MC works for a full involvement of family and community fighting the dangerous stigma linked to dementia.
The principal goal of our protocol was to create a multi-component system that provides a quick and satisfactory access to clinical services. By a standardized re-evaluation of clinical, psychological and social conditions and a personalized care planning, we want to resolve issues related to the emergent problems in the dementia’s course. This aspect is very important because it identifies timely any underlying problems (physical, cognitive behavioral and social changes). Recently, the transitional care for people with chronic disease has gained great importance [48]. Caring for PwD in acute setting is challenging especially when the acute phase (see for example pneumonia infection) is overcome and the patients must be discharged. Often their family are frightened about the care load after an acute event that worsen the previous frail balance of their loved one. For this reason, it is mandatory to define new and appropriate modality to manage some fundamental phases that are still fragmentary and not sufficiently coordinated. After reaching a complete consensus, our experts have identified the crucial phases in the disease management of persons living with cognitive impairment (Table 1). This project has integrated different professional figures and departments for an articulated intervention plan. It is important to raise awareness in social community and among GPs who are the first detector of any suspicious change in the cognitive performance of our patients. As dementia is a progressive chronic condition that impair the ability to live independently, it is reasonable to assume that PwD keep close contact with their own GP firstly [49]. In fact, family physicians are generally patients’ first point of contact with the health system. Further, they are positioned ideally to provide care for individuals living with dementia from the early to the end stages of the illness [50,51]. Sometimes, the caregiver’s perception does not go in this direction [52]. This is why we pressed that specialists or social workers in the acute setting to keep contact with the GP constantly.
Dementia is a dyad-disease because it affects both patients and their family. The size of the problem, however, is such that a broad approach is needed. A comprehensive response by the health and social care system (both public and private) and other government sectors is mandatory. Moreover, we are convinced that a more and structured dementia care pathway should be integrated in the health community. In fact, it is important to provide some comprehensive, multisector policy responses to improve quality of life and reduce stigma and social isolation for PwD and their caregivers.
Another important aspect is that the residential living or day care for PwD often are expensive. The number of sufferers was estimated to increase more and more and the retirement benefits probably will not cover the health cost entirely (especially in some country as in Italy where politic situation has influenced the household economic power hitting the weakest groups of our society). Consequently, to meet these needs and demands, it will be important to develop centers that could coordinate any aspect of this multifaceted disease (both the clinical and the social features).
In our project, the MC will be responsible to maintain partnership with Adult day care, Alzheimer’s Café, and with the municipal social workers. Social networks are important in the prevention and in the management of dementia [53]. A center staffed by clinical providers, social workers, and volunteers could offer an opportunity for leisure activities and social support to PwD. In fact, several studies have demonstrated the usefulness of a variety of non-pharmacological approaches to reduce the negative effects of giving care to PwD. Nevertheless, the current scientific evidence is conflicting. Sometimes, caregivers provide care with little or no support [54,55,56], while suffering from poor health themselves [56]. In fact, family members who provide care to individuals with chronic or disabling conditions are themselves at risk. Emotional, mental, and physical health problems arise from complex caregiving situations [57]. For this reason, it is necessary to consider the importance of psychological groups for caregiver support. Brodaty and colleagues stated that most caregivers are satisfied with the educational programs they have attended reporting an improvement in their relationship with the patient [58]. Based on this evidence, we have established some psychological family groups with two meetings monthly. We collaborate with territorial services, volunteers, association groups to grow-up the social networks promoting the social activities. In our project, PwD are not excluded from the own social life and we try to avoid the social stigma of this disease.
Dementia is a standing concern around the world. Despite convincing evidence on the pathogenesis and epidemiology of dementia, so far we know little about the experiences of people with cognitive disorders in the health system and how the health system is modeled on them. Some examples of dementia care pathway [15,59] such as the Dementia Care Pathway in New Zealand [60] and in Australia [61] provided an attempt to meet the needs of PwD. They offer a guidance across various services but only recommendations for acute care. By the evaluation of the current practice, they identified barriers and facilitators for the implementation of the best practice in dementia care. However, there is not a coordinated and integrated vision to join acute, chronic and community setting as in our project. Some studies explored the current state of health assistance for older people with dementia in the acute care setting but without mention to the integrated care pathway. Although there was an increase in the development and use of these pathways in the acute settings [62,63,64], however some concerns have emerged. In fact, there is still an insufficient evidence to support the effectiveness of these pathways in the acute setting and more studies should be encouraged.
This project has several strengths. Firstly, the possibility in our acute setting to keep close contact with various agencies and department in care provision. Secondly, the possibility to create a definite safety net for PWD and their family by the simultaneous activation of more interventions. Potentially, this could prevent some possible breakdowns in the healthcare system. In fact, because dementia is a chronic, disabling and devastating disease, it affects the healthcare expenditure enormously, especially where there is not a priori care program. Thirdly, our pathway assesses, engages, and supports caregivers (Figure 1). It is well-known that every intervention performed in order to reduce the caregiver’s distress influence the behavior and well-being of PwD positively [65]. For this reason, we wanted to provide to caregivers effective tools to reduce their distress through psychological support groups, explaining with clarity what could happen during the life course in PwD. Moreover, we give them the possibility to keep close contact with the CDCD (they can write in any moment to the CDCD’s specialist).
Finally, we believe that communication between clinical providers is essential to intercept early cognitive impairment cases. We have promoted an open approach to the health system through a close involvement of general practitioners. In fact, general practitioners are the first doctor with whom people deal with their health problems. However, gaps in symptom recognition and initiation of cognitive tests could lead to diagnostic delay or improper interpretation. Frequently, there is no communication between care providers, specialists and primary care physicians. This lack of connection is a weaker link to disease detection [66]. Hospitals are full of dementia screening resources, but time constraints and serious medical problems are preventing an efficient diagnosis of dementia. The memory clinics offer access to multidisciplinary skills (neurologist, geriatric and neuropsychologist), demonstrating an early diagnosis of dementia and a potential cost-effectiveness, but they are disadvantaged by long waiting lists. Once the diagnosis is made, it is necessary to follow the PWD, but sometimes it is not easy to have timely access to clinical services. Dementia is a very common and increasing disease and it is very important to create a multiple network that includes general practitioners, hospitals for acute illnesses, specialized clinics, communities and nursing homes. Governments have been called to provide good quality care and services for PWD. Countries were asked to shape and share solutions to provide facilities through the continuum of health care. Furthermore, it is mandatory to ensure both the development and training of a harmonized workforce able to counteract the huge impact of this disease.
4. Conclusions
The aging population, the changes in household structure, the increase of immigration rate (linked to a new class of elderly in the future), and the economic crisis, all of these are contributing factors that must encourage to develop and implement of strategies for welfare support. Despite an extensive research in the field of dementia, deficits in the quality of dementia care still exist. There is a paucity of robust research concerning the care experiences for PwD, although several countries have yet to implement the ICPs in their settings. The interpretation of existing ICPs requires careful consideration and cautious judgements on their suitability and feasibility. In our opinion, an economic evaluation and a better definition of outcomes could enhance the quality and the spreading out of this care pathway for PwD. It is mandatory to promote these experiences if we want to analyze some impactful policies improving services for PwD and their family.
Although the international and national guidelines drive how the care trajectory should be, unfortunately at the local level the correct procedures are often missed because the bureaucratic problems and the paucity of resources interfere with the guidelines application. In fact, without a well-structured, real based action plan against dementia we could assist to a collapse of our healthcare system. It follows that the local governments might encounter in the real life different limitations to the application of the ideal project, so we need to share and cope with them; otherwise, we are going to fail into manage the dementia syndrome over time. Where no cure is still available, we have to bet on the care system.
Actually, the true problem is a lack of organization among clinical and social providers who have to co-operate for the same goal, that is, to ensure the best, personalized and appropriate pathway of care for PwD. However, providers struggle with some problems as the time constraints, coordination of care, and a limited clinical support system, often times resulting in care outcomes that are less than personally desirable and satisfying.
Implementing dementia care pathway in the hospital setting, as shown in our experience, is a potential means of improving care, enabling existing services to work in a more unified way. This operational protocol set up the interventions to enable the healthcare system providing true and qualified care for PwD. Everyone belonging to the ICP will receive supports and treatments that are targeted to meet its individual requirements. Furthermore, thanks to a whole cooperation of all above-mentioned actors in this pathway, it is possible to improve the transition between community and acute setting for PwD. Whether the ICP for dementia is a useful method for the care process or not, it must still be evaluated. Therefore, it is very important to promote further studies centered on PwD that explore the best method for ethical and sustainable health use. Our future efforts are heading in this direction.
Acknowledgments
We want to thank the research team of “Dementia Care Pathway” of ASST “Carlo-Poma” Hospital and all of the associations and Nursing Home that help us to give the best support to our patients and their families.
We want to thank Savina from “Fondazione Mons. Arrigo Mazzali – ONLUS” together to all colleagues that are working there.
We thank Dr. Suheil Abdul Ponnambath for his help in English revision of our manuscript
Finally, we thanks our patients and their families because they give us a challenge to cope with every day.
Author Contributions
V. Frisardi, contributed to the analysis of the results and to writing of the manuscript.
F. Biagi, E. Galante, A. Balzanelli, E. Talassi and M.C Cilia perform the neuropsychological assessments of patients and provide to data collection.
G. Capiluppi, she is a nurse of the MC and she contributed to help GP and people to have an easy access to dementia care pathway; she filled out evaluation scale for monitoring patients at home.
L. Frittoli is a case officer of CDCD and she contributed to enroll the patients and to keep close contact with them, Further, she encouraged V Frisardi to rationalize this project and supervised the findings of this work.
D. Terzi; E.Podavini G. Gazzoni, G. Simoncelli have cooperated with V. Frisardi and they supervised the findings of this work.
S Faroni, A. Taragnani, worked to promote this project and ameliorated the dementia care pathway.
C Matarazzo, M Avesani, C.M Stucchi discussed the results and contributed to visit patients.
A. Ciccone, Balzanelli A. Ventura A., Terzi D. Taragnani A, Bellani, Galante E, Biagi F, Talassi E, C. Basili, M.Galavotti, contributed to the design and implementation of the research.
Funding
This project is inserted in the national plan for dementia.
Competing Interests
The authors have declared that no competing interests exist.
References
- Connolly A, Gaehl E, Martin H, Morris J, Purandare N. Underdiagnosis of dementia in primary care: Variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health. 2011; 15: 978-984 [CrossRef] [Google scholar] [PubMed]
- World Alzheimer Report [WHO] 2015 The Global Impact of Dementia. Available from: www.worldalzreport2015.org/downloads/world-alzheimer-report-2015. [Google scholar]
- Niu H, Álvarez-Álvarez I, Guillén-Grima F, Aguinaga-Ontoso I. Prevalence and incidence of Alzheimer's disease in Europe: A meta-analysis. Neurologia. 2017; 32: 523-532. [CrossRef] [Google scholar] [PubMed]
- Alzheimer’s Disease International. Policy Brief for Heads Of Government. The global Impact of Dementia 2013-2050. London. Available from: http://www.alz.co.uk/research/GlobalImpacDementia2013. October 14,2014.
- Global action plan on the public health response to dementia 2017 – 2025. WHO. Available from: http://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/
- Agüero-Torres H., Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998; 88: 1452-1456. [CrossRef] [Google scholar] [PubMed]
- Skinner T, Scott I, Martin J. Diagnostic errors in older patients: A systematic review of incidence and potential causes in seven prevalent diseases Int J Gen Med. 2016; 9: 137-146. [CrossRef] [Google scholar] [PubMed]
- Hope T, Keene J, Fairburn CG, Jacoby R, McShane R. Natural history of behavioural changes and psychiatric symptoms in Alzheimer's disease. A longitudinal study. Br J Psychiatry. 1999; 174: 39-44. [CrossRef] [Google scholar] [PubMed]
- Maust DT, Kales HC, McCammon RJ, Blow FC, Leggett A, Langa KM. Distress associated with dementia-related psychosis and agitation in relation to healthcare utilization and costs. Am J Geriatr Psychiatry. 2017; 25: 1074-1082. [CrossRef] [Google scholar] [PubMed]
- Sampson EL, White N, Leurent B, Scott S, Lord K, Round J, et al. Behavioural and psychiatric symptoms in people with dementia admitted to the acute hospital: Prospective cohort study. Br J Psychiatry. 2014; 205: 189-196. [CrossRef] [Google scholar] [PubMed]
- Sleeman KE, Perera G, Stewart R, Higginson IJ. Predictors of emergency department attendance by people with dementia in their last year of life: Retrospective cohort study using linked clinical and administrative data. Alzheimers Dement. 2018; 14: 20-27. [CrossRef] [Google scholar] [PubMed]
- Lillo-Crespo M, Riquelme J, Macrae R, De Abreu W, Hanson E, Holmerova I, et al. Experiences of advanced dementia care in seven European countries: Implications for educating the workforce. Glob Health Action. 2018; 11: 1478686. [CrossRef] [Google scholar] [PubMed]
- Schrijvers G, van Hoorn A, Huiskes N. The care pathway: Concepts and theories: An introduction. Int J Integr Care. 2012; 12: e192. [CrossRef] [Google scholar] [PubMed]
- Vanhaecht K, Panella M, van Zelm R, Sermeus M. An overview on the history and concept of care pathways as complex interventions. SAGE. 2010; 14: 117-123. [CrossRef] [Google scholar]
- National Institute of Clinical Excellence Pathways. Dementia: Supporting people with dementia and their carers in health and social care. Available from: https://www.nice.org.uk/guidance/cg42.
- Di Fiandra T, Canevelli M, Di Pucchio A, Vanacore N. Italian dementia national plan working group. The Italian dementia national plan. Commentary. Ann Ist Super Sanita. 2015; 51: 261-264. [Google scholar]
- Goodman D, Ogrinc G, Davies L, Baker GR, Barnsteiner J, Foster TC, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016; 25: e7. [CrossRef] [Google scholar] [PubMed]
- Keeney S, Hasson F, Mckenna H. The delphi technique in nursing and health research. West Sussex: John Wiley & Sons Ltd; 2010. [Google scholar]
- Lezak M, Howieson D, Loring D. Neuropsychological assessment. New York: Oxford University Press; 2004. [Google scholar]
- Strauss E, Sherman E, Spreen O. A compendium of neuropsychological tests. New York: Oxford University Press; 2006. [Google scholar]
- Barletta-Rodolfi C, Gasparini F, Ghidoni E. Kit del neuropsicologo italiano. Milano: Dynamicon; 2011. [Google scholar]
- MF Folstein, SE Folstein, PR McHugh. Mini Mental State Examination. J Psychiat Res. 1975; 12: 189-198. [CrossRef] [Google scholar] [PubMed]
- Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, et al. The frontal assessment battery (FAB): Normative values in an Italian population sample. Neurol Sci. 2005; 26: 108-116. [CrossRef] [Google scholar] [PubMed]
- Monaco M, Costa A, Caltagirone C, Carlesimo GA. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol Sci. 2013; 34: 749-754. [CrossRef] [Google scholar] [PubMed]
- Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google scholar]
- Della Sala S, Laiacona M, Spinnler H, Ubezio C. A cancellation test: Its reliability in assessing attentional deficits in Alzheimer's disease. Psychol Med. 1992; 22: 885-901. [CrossRef] [Google scholar] [PubMed]
- Abbate C, Luzzatti C. Vergani C. Matrix test: Speed and accuracy of visual search in aging. G Gerontol. 2007; 55: 11-20. [Google scholar]
- Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: Normative values from 287 normal adult controls. Ital J Neurol Sci. 1996; 17: 305-309. [CrossRef] [Google scholar] [PubMed]
- Costa A, Bagoj E, Monaco M, Zabberoni S, De Rosa S, Papantonio AM, et al. Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol Sci. 2014; 35: 365-372. [CrossRef] [Google scholar] [PubMed]
- Tessari A, Tornalo A, Lunardelli A, Zadini A, Rumiati RI. STIMA: A short screening test for ideo-motor apraxia, selective for action meaning and bodily district. Neurol Sci. 2015; 36: 977-984. [CrossRef] [Google scholar] [PubMed]
- Cummings JL, Mega M, Gray K, Rosemberg-Thompson S, Carusi DA, Gornbei J. Neuropsychiatric Inventory (NPI). Neurology. 1994; 44: 2308-2314. [CrossRef] [Google scholar] [PubMed]
- Katz S., Akpom C. A. Index of ADL. Med Care. 1976; 14: 116-118. [CrossRef] [Google scholar] [PubMed]
- Lawton MP. Instrumental activities of daily living. Gerontologist. 1969; 9: 179. [CrossRef] [Google scholar]
- Negri L, Piazza G, Sartori RDG, Cocchi MG, Delle Fave A. The adult carer quality of life questionnaire (AC-QoL): Comparison with measures of burden and well-being, and Italian validation. Disabil Rehabil. 2018; 10: 1-10. [CrossRef] [Google scholar] [PubMed]
- Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989; 29: 798-803. [CrossRef] [Google scholar] [PubMed]
- O'Connor ML, McFadden SH. Development and psychometric validation of the dementia attitudes scale international. Int J Alzheimers Dis. 2010; 2010: 454218. [Google scholar]
- Greene JG, Smith R, Gardiner M, Timbury GC. Measuring behavioural disturbance of elderly demented patients in the community and its effects on relatives: a factor analytic study. Age Ageing. 1982; 11: 121-126. [CrossRef] [Google scholar] [PubMed]
- Teo N, Gao Q, Nyunt MSZ, Wee SL, Ng TP. Social frailty and functional disability: findings from the Singapore longitudinal ageing studies. J Am Med Dir Assoc. 2017; 18: 637. [CrossRef] [Google scholar] [PubMed]
- Pirani A, Brodaty H, Martini E, Zaccherini D, Neviani F, Neri M. The validation of the Italian version of the GPCOG (GPCOG-It): A contribution to cross-national implementation of a screening test for dementia in general practice. Int Psychogeriatr. 2010; 22: 82-90. [CrossRef] [Google scholar] [PubMed]
- Yokomizo JE, Simon SS, Bottino CM. Cognitive screening for dementia in primary care: A systematic review. Int Psychogeriatr. 2014; 26: 1783-1804. [CrossRef] [Google scholar] [PubMed]
- Multiservice center of ASST Mantova. Available from: http://www.asst-mantova.it/centro-multiservizi.
- Cole MG. Delirium in elderly patients. Am J Geriatr Psychiatry. 2004; 12: 7-21. [CrossRef] [Google scholar]
- Inouye SK. Cognitive decline: Strategies for prevention: Proceedings of a white house conference on aging. London: Greenwich Medical Media; 1997. [Google scholar]
- Electronic Informative Healthcare System of ASST Carlo Poma. Available from: http://www.siss.regione.lombardia.it/wps/portal/Minisiti/siss.
- A Measurement and Performances Rating System. MAntua Intranet Ats (MAIA) System website. Available from: https://www.aslmn.net/
- Jing W, Willis R, Feng Z. Factors influencing quality of life of elderly people with dementia and care implications: A systematic review. Arch Gerontol Geriatr. 2016; 66: 23-41. [CrossRef] [Google scholar] [PubMed]
- Herron RV, Rosenberg MW. "Not there yet": Examining community support from the perspective of people with dementia and their partners in care. Soc Sci Med. 2017; 173: 81-87. [CrossRef] [Google scholar] [PubMed]
- Ray CA, Ingram V, Cohen-Mansfield J. Systematic review of planned care transitions for persons with dementia. Neurodegener Dis Manag. 2015; 5: 317-331. [CrossRef] [Google scholar] [PubMed]
- Moore A, Frank C, Larry WC. Role of the family physician in dementia care. Can Fam Physician. 2018; 64: 717-719. [Google scholar]
- Moore A. Fourth canadian consensus conference on the diagnosis and treatment of dementia. Recommendations for family Physicians. Can Fam Physician. 2014; 60: 433-438. [Google scholar]
- Villars H, Oustric S, Andrieu S, Baeyens JP, Bernabei R, Brodaty H, et al. The primary care physician and Alzheimer’s disease: an international position paper. J Nutr Health Aging. 2010; 14: 110-120 [CrossRef] [Google scholar] [PubMed]
- Schoenmakers B, Buntinx F, Delepeleire J. What is the role of the general practitioner towards the family caregiver of a community-dwelling demented relative? A systematic literature review. Scand J Prim Health Care. 2009; 27: 31–40. [CrossRef] [Google scholar] [PubMed]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004; 3: 343-353. [CrossRef] [Google scholar] [PubMed]
- Alzheimer’s Association & National Alliance for Caregiving. Families Care: Alzheimer’s Caregiving in the United States. Chicago: Alzheimer’s Association and Bethesda; 2004. [Google scholar]
- Family Caregiver Alliance Caregiver Assessment: Principles, Guidelines and Strategies for Change. Report from a National Consensus Development Conference (Vol. I). (2006). [Google scholar]
- Navaie-Waliser M, Feldman PH, Gould DA, Levine CL, Kuerbis AN, Donelan K. When the caregiver needs care: The plight of vulnerable caregivers. Am J Public Health. 2002; 92: 409-413. [CrossRef] [Google scholar] [PubMed]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. Gerontologist. 1990; 30: 583-594. [CrossRef] [Google scholar] [PubMed]
- Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial intervention for caregivers of people with dementia. J Am Geriatr Soc. 2003; 51: 657-664. [CrossRef] [Google scholar] [PubMed]
- Dementia Strategy NHS Forth Valley. Available from: https://nhsforthvalley.com/.../Dementia-Strategy-2017-2020
- New Zealand Framework for Dementia Care - Ministry of Health. Available from: https://www.health.govt.nz/publication/new-zealand-framework-dementia-care
- Tropea J, LoGiudice D, Brand C. The Dementia Care Pathway for use in acute care. Available from: https://medicine.unimelb.edu.au/research-groups/medicine-and-radiology-research/royal-melbourne-hospital/melbourne-epicentre/the-dementia-care-pathway-for-use-in-acute-care.
- Moyle W, Borbasi S, Wallis M, Olorenshaw R, Gracia N. Acute care management of older people with dementia: a qualitative perspective. J Clin Nurs. 2011; 20: 420-428. [CrossRef] [Google scholar] [PubMed]
- Butcher L. Caring for patients with dementia in the acute care setting. Br J Nurs. 2018; 27: 358-362. [CrossRef] [Google scholar] [PubMed]
- Handley M, Bunn F, Goodman C. Dementia-friendly interventions to improve the care of people living with dementia admitted to hospitals: a realist review. BMJ Open. 2017; 7: e015257. [CrossRef] [Google scholar] [PubMed]
- Feast A, Moniz-Cook E, Stoner C, Charlesworth G, Orrell M. A systematic review of the relationship between behavioral and psychological symptoms (BPSD) and caregiver well-being. Int Psychogeriatr. 201; 28: 1761-1774. [CrossRef] [Google scholar] [PubMed]
- Marshall M, Phillips D. A qualitative study of the professional relationship between family physicians and hospital specialists. Prof Geogr. 1999; 51: 274-282. [CrossRef] [Google scholar]


