Interphase FISH: A Helpful Assay in Prenatal Cytogenetics Diagnosis
Elena Sala 1, * ![]() , Donatella Conconi 2
, Donatella Conconi 2 ![]() , Francesca Crosti 1
, Francesca Crosti 1 ![]() , Nicoletta Villa 1
, Nicoletta Villa 1 ![]() , Serena Redaelli 2
, Serena Redaelli 2 ![]() , Gaia Roversi 1, 2
, Gaia Roversi 1, 2 ![]()
- Medical Genetics Laboratory, San Gerardo Hospital, Monza, Italy
- School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy
OBM Genetics 2019, Volume 3, Issue 1 doi: 10.21926/obm.genet.1901063
Academic Editor: Thomas Liehr
Special Issue: Applications of Fluorescence in Situ Hybridization
Received: October 01, 2018 | Accepted: January 21, 2019 | Published: January 29, 2019
Recommended citation: Sala E, Conconi D, Crosti F, Villa N, Redaelli S, Roversi G. Interphase FISH: A Helpful Assay in Prenatal Cytogenetics Diagnosis. OBM Genetics 2019; 3(1): 063; doi:10.21926/obm.genet.1901063.
© 2019 by the authors. This is an open access article distributed under the conditions of the Creative Commons by Attribution License, which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is correctly cited.
Abstract
Since its introduction around the end of the 1970s, interphase fluorescence in situ hybridization (FISH) supports both classical and recent techniques for determining fetal karyotypes during prenatal diagnosis, quickly providing relevant information for the management of pregnancy. Interphase FISH plays an important role in the study of pregnancies with malformations, in mosaicism conditions, in confirming or excluding aneuploidy detected by non-invasive prenatal testing, and in the diagnosis of contiguous gene syndromes due to microdeletions. The availability of many commercial probes from different genomic regions makes this method versatile and easy to apply in the pathways of prenatal diagnosis and fetal care.
Graphical abstract
Keywords
Prenatal diagnosis; interphase FISH; array-CGH; NIPT
1. Introduction
Since its introduction around the end of the 1970s [1,2], in situ hybridization has proven to be a reliable technique for the detection of DNA sequences and localization on both nuclei and chromosomes. Over the years, the advent of probes directly labelled with different fluorochromes reduced overall test time and also introduced the possibility of multiple probes in the same experiment (multicolor hybridization).
The greater variety of the currently available probes (whole and partial chromosome painting; gene-, centromere-, or telomere-specific; break-apart; etc.) makes this technique extremely versatile and permits its use in different phases of diagnostic pathways.
Together with the classic application on chromosomes in metaphase, the use of fluorescence in situ hybridization (FISH) on interphase nuclei has allowed the analysis of specific DNA sequences in samples that would have otherwise been difficult to investigate, such as paraffin-embedded tissues or tissues for which it is difficult to obtain metaphases. In particular, in the prenatal context, the use of interphase FISH is an excellent tool for the complex diagnosis of fetal chromosomal abnormalities, playing a vital role in the decision making process of pregnant women.
Between 1990 and 2000, several publications focused interest on the possibility of using interphase FISH in cases of fetal malformations. The possibility that a screening test could identify a possible risk of fetal chromosome alteration induces a particular emotional state in the patient; a shorter time required for diagnosis enables a faster decision with a consequent reduction of anxiety [3].
From this perspective, interphase FISH with specific probes for chromosomes 13, 18, 21, X, and Y allows identification of 60-80% of the chromosomal abnormalities present at birth [4,5], reducing the diagnosis time from a conventional 7-10 days to 24-48 hours. In the presence of normal ploidy for these chromosomes, the overall residual risk of chromosomal alteration is considerably reduced, especially in the subgroup of women with advanced maternal age or anxiety (Table 1).
Table 1 Residual risk for a cytogenetic abnormality after a normal FISH result.

Fetal karyotyping as a diagnostic tool underwent profound changes with the introduction of array-CGH and non-invasive screening tests. The molecular karyotype obtained by array-CGH increases the diagnostic power of the conventional karyotype. Moreover women, especially in low risk categories of fetal chromosomal abnormalities, choose non-invasive screening tests, based on detection of fetal DNA in maternal blood.
Despite the introduction of these new methods, karyotype–phenotype correlation in prenatal diagnosis can be difficult in some complex issues, such as the presence of low-level chromosomal mosaicism or mosaicism confined to the placenta.
Also, in regard to contiguous gene syndromes for which FISH is necessary to confirm an ultrasound suspicion, a fast fetal karyotype result permits a better management of pregnancy.
Interphase FISH complements all these pathways well, thanks to the large availability of specific commercially-available probes for diagnostic use, which overcomes the problems associated with the use of "homemade" probes. This includes having quality control probes and a positive control probe for each experiment in order to evaluate the accuracy of the technical procedure.
2. Interphase FISH and Array-CGH
The fetal karyotype analysis in prenatal diagnosis is a powerful method for detecting most chromosomal anomalies, like aneuploidies and balanced or unbalanced chromosomal rearrangements, with a resolution of 10-15Mb, depending on the quality of metaphases. Array-CGH is a high-throughput method that can detect copy number variations throughout the genome to a resolution of 5 Kb for SurePrint G3 400K arrays (Agilent). In the last few years, its introduction in prenatal diagnosis has received considerable impetus, mostly in pregnancies with ultrasound fetal malformations, until it was considered the most suitable diagnostic test in this subgroup of patients. Several authors have shown that the use of array-CGH increases the identification of unbalanced genomic alterations of relevant clinical significance in about 5.2% in pregnancies with malformations that include cardiac abnormalities, pathological nuchal translucency, cystic hygroma, hydrops, or central nervous system anomalies [9].
However, the high costs of the array-CGH technique, its uselessness in the absence of a clear gain or loss of chromosomal regions, and the difficulty in identifying mosaicisms below 30% are the main factors that inhibit its replacement of the conventional karyotype.
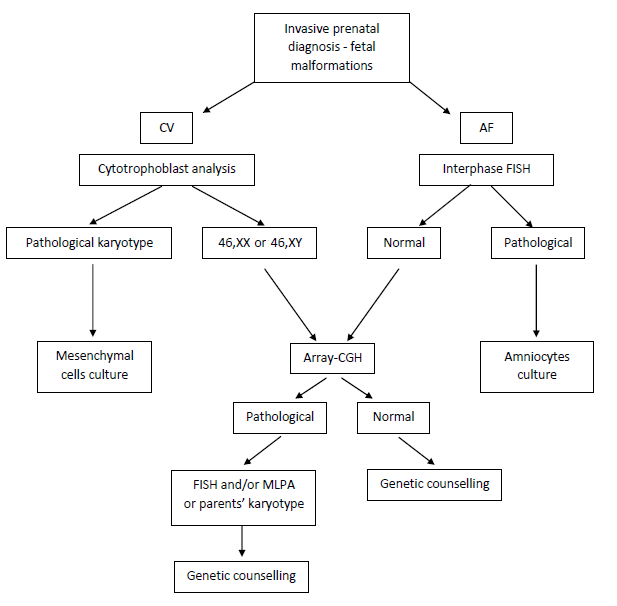
Use of interphase FISH with probes for the detection of chromosomes 13, 18, 21, X, and Y aneuploidies, especially in pregnancies that have ultrasound malformations, identifies the majority of chromosomal alterations present at birth, designating pregnancies with normal interphase FISH to the more complex study by array-CGH. The following flow chart outlines the algorithm used in our laboratory.

Figure 1 Diagnostic flow chart. Algorithm used in our laboratory. CV: chorionic villi; AF: amniotic fluid.
3. Interphase FISH and NIPT
In recent years, the use of non-invasive prenatal testing (NIPT) that analyzes cell-free fetal DNA (cffDNA) in maternal circulation has been greatly developed. This methodology shows excellent performance in detecting the most common aneuploidies, even if the PPV (positive predictive value, that is, the percentage of patients with a positive test who actually have the chromosomal abnormality) declines significantly for trisomy 13 and monosomy or trisomy X [10]. Methodological limitations are observed in twin pregnancies, fetoplacental mosaicism, vanishing twin syndrome, and maternal chromosomal abnormalities [11].
As currently practiced, this method is also used for the identification of rare autosomal trisomies and the most common microdeletions. Fetal DNA isolated from maternal blood originates from trophoblasts, and therefore, these tests are accompanied by long-known problems concerning placental mosaicism and fetal/placental discrepancy. Interphase FISH is successful in confirming or excluding aneuploidy detected by NIPT, both rapidly and accurately, given the possibility that the analysis could consist of a large number of nuclei that have not undergone any in vitro growth processes (culture artifacts).
Selected cases:
Case 1
NIPT was performed at the 11th week of gestation on a 38-year-old woman referred for advanced maternal age. The fetal DNA fraction was 13% and the result was consistent with the presence of fetal monosomy X. Amniocentesis was performed at the 16th week of gestation and interphase FISH with chromosomes 18, X, and Y alpha satellite probes revealed disomic nuclei in 100 nuclei scored (Figure 2A). Interphase FISH analysis with probes specific for chromosomes 13 and 21 also revealed disomic nuclei (Figure 2B). Chromosomal analysis on metaphases showed a normal female karyotype (46,XX).
Case 2
A 36-year-old woman underwent NIPT for advanced maternal age. Fetal DNA fraction was 10% and the result was compatible with the presence of fetal trisomy 18. No fetal ultrasound abnormalities were identified. Amniocentesis was performed at the 16th week of gestation and interphase FISH with chromosomes 18, X, and Y alpha satellite probes revealed disomic nuclei in 100 nuclei scored (Figure 2C). Interphase FISH analysis with probes specific for chromosomes 13 and 21 also revealed disomic nuclei (Figure 2D). Chromosomal analysis on metaphases showed a normal female karyotype (46,XX).
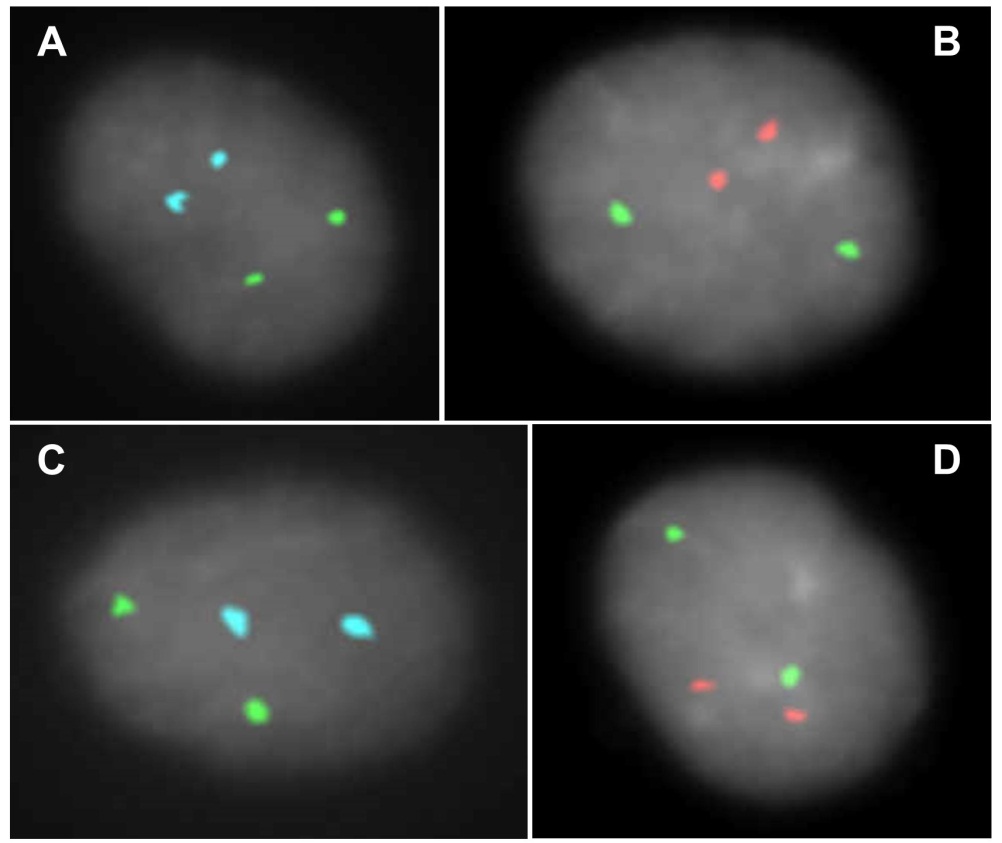
Figure 2 FISH analyses of cases 1 and 2. A: Interphase FISH with commercial alpha satellite probes specific for chromosomes 18 (aqua), X (green), and Y (red; absent in these nuclei) (CytoCell). B: Interphase FISH with the commercial probes specific for chromosomes 13 (green) and 21 (red) (CytoCell). C: Interphase FISH with commercial alpha satellite probes specific for chromosomes 18 (aqua), X (green), and Y (red; absent in these nuclei) (CytoCell). D: Interphase FISH with commercial probes specific for chromosomes 13 (green) and 21 (red) (CytoCell).
4. Interphase FISH and Mosaicism
Chromosomal mosaicism is the presence of two or more cell lines in the same individual. The detection of several cell lines with different karyotypes in prenatal diagnosis generally requires the analysis of a large amount of cells from different cultures and/or different tissues, to better define the observed alteration [12,13]. Approximately 1%-2% of prenatal diagnoses from chorionic villus samples exhibit mosaicism or fetal/placental discrepancy [14]: these cases require further study for the correct definition of fetal karyotype and management of pregnancy. The detection of mosaicism in amniocentesis is around 0.2% of the samples [15]. The validation of mosaicism took place in the past through the analysis of the fetal blood karyotype obtained by cordocentesis. To date, this procedure is rarely performed due to its invasiveness, the high miscarriage risk, and the need of expertise to perform the procedure. Moreover, fetal blood culture is not a good tissue source for mosaic analysis of some chromosomes [16,17]. The availability of specific centromeric probes for almost every chromosome allows the use of interphase FISH on uncultured amniocytes for the study of mosaics in prenatal diagnosis, combining rapid diagnosis with the possibility of analyzing a large number of nuclei deriving from different fetal tissues.
Selected cases:
Case 3
A 40-year-old woman underwent chorionic villi sampling at the 14th week of gestation for advanced maternal age. The karyotype on direct CVS preparation (short-term culture) evidenced two cellular lines: one with a normal male karyotype (46,XY; 6 metaphases) and one with a trisomy of chromosome 13 (47,XY,+13; 6 metaphases) as seen in Figure 3A. Mesenchymal long-term culture showed only a normal male karyotype (46,XY).
Interphase FISH analysis of amniotic fluid cells with chromosomes 13 and 21 probes revealed only disomic nuclei in 100 nuclei scored (Figure 3B). Chromosomal analysis on metaphases showed a normal male karyotype (46,XY).
Case 4
A 38-year-old woman underwent amniotic fluid sampling at the 16th week of gestation for advanced maternal age. The chromosomal analysis revealed the presence of one clone with trisomy 11 (47,XY,+11) as seen in Figure 3C, and 12 clones from 4 independent cultures (in situ chromosomal preparations) with a normal male karyotype (46,XY).
Interphase FISH analysis on a second sample of uncultured amniotic fluid nuclei with a chromosome 11 alpha satellite probe (D11Z1) showed disomic nuclei in 100 nuclei scored (Figure 3D). Uniparental disomy for chromosome 11 was excluded.
Case 5
A 44-year-old woman underwent amniotic fluid sampling at the 16th week of gestation for advanced maternal age. The chromosomal analysis revealed the presence of 4 metaphases from two independent cultures with trisomy 7 (47,XY,+7) as seen in Figure 3E, and 21 metaphases from 3 independent cultures with a normal male karyotype (46,XY); chromosomal preparations were made after culture trypsinization.
Interphase FISH analysis on a second sample of uncultured amniotic fluid nuclei with a chromosome 7 alpha satellite probe (CEP7), as well as a chromosome 3 alpha satellite probe (CEP3) that was used as internal control, showed disomic nuclei in 100 nuclei scored (Figure 3F). Uniparental disomy for chromosome 7 was excluded.
Case 6
A 38-year-old woman underwent chorionic villi sampling at the 15th week of gestation for advanced maternal age. The karyotype on direct CVS preparation (short-term culture) evidenced two cellular lines: the first with a normal female karyotype (46,XX; 22 metaphases) and the second with a trisomy of chromosome 8 (47,XX,+8; 8 metaphases) as seen in Figure 3G. Mesenchymal long-term culture showed only a normal female karyotype (46,XX).
Interphase FISH analysis on uncultured amniotic fluid cells with LSI IGH, LSI MYC, and CEP8 probes (specific probes for IgH locus, MYC locus, and chromosome 8, respectively) revealed disomic nuclei in 100 nuclei scored (Figure 2H). Chromosomal analysis on metaphases showed a normal female karyotype (46,XX).
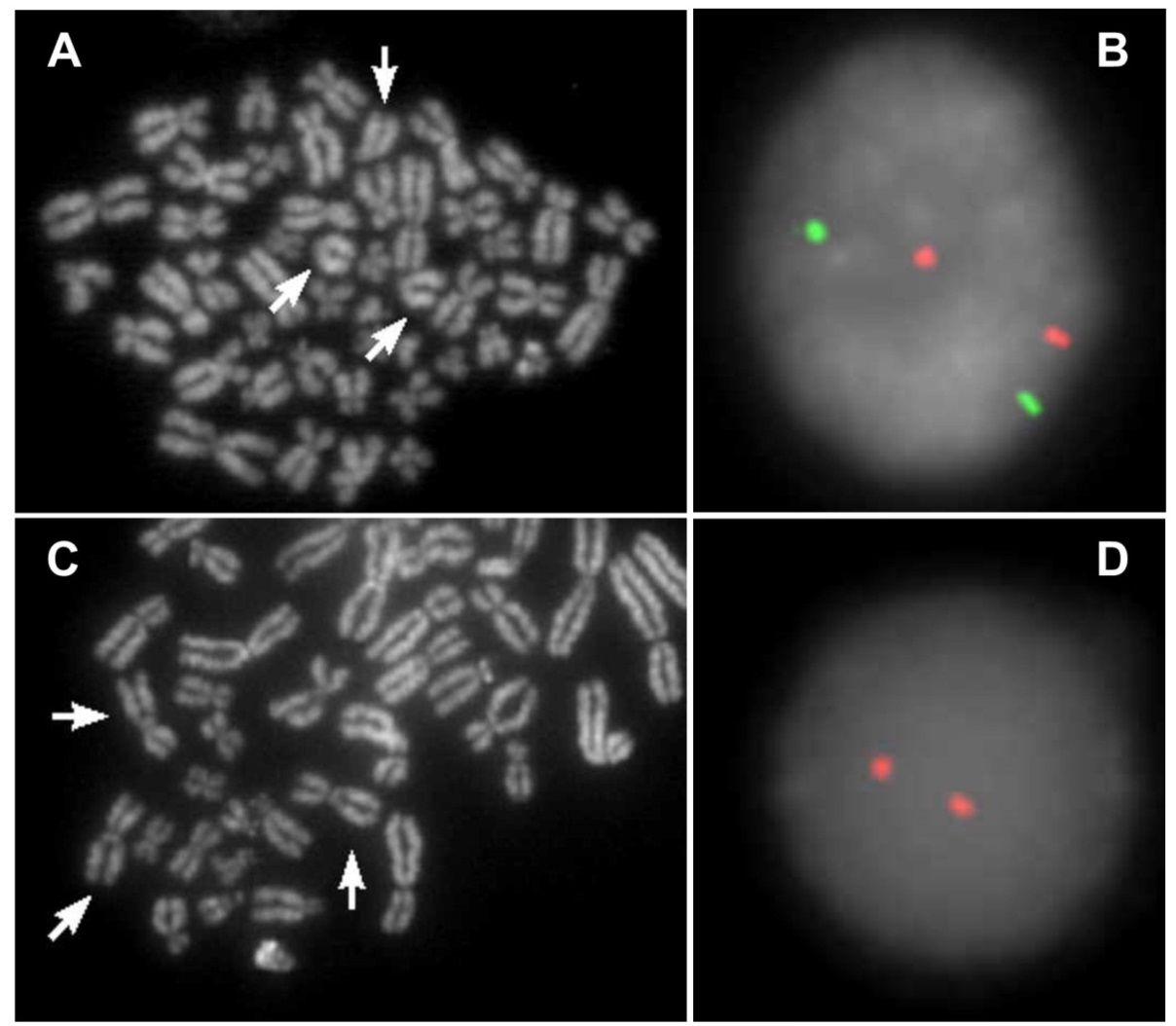
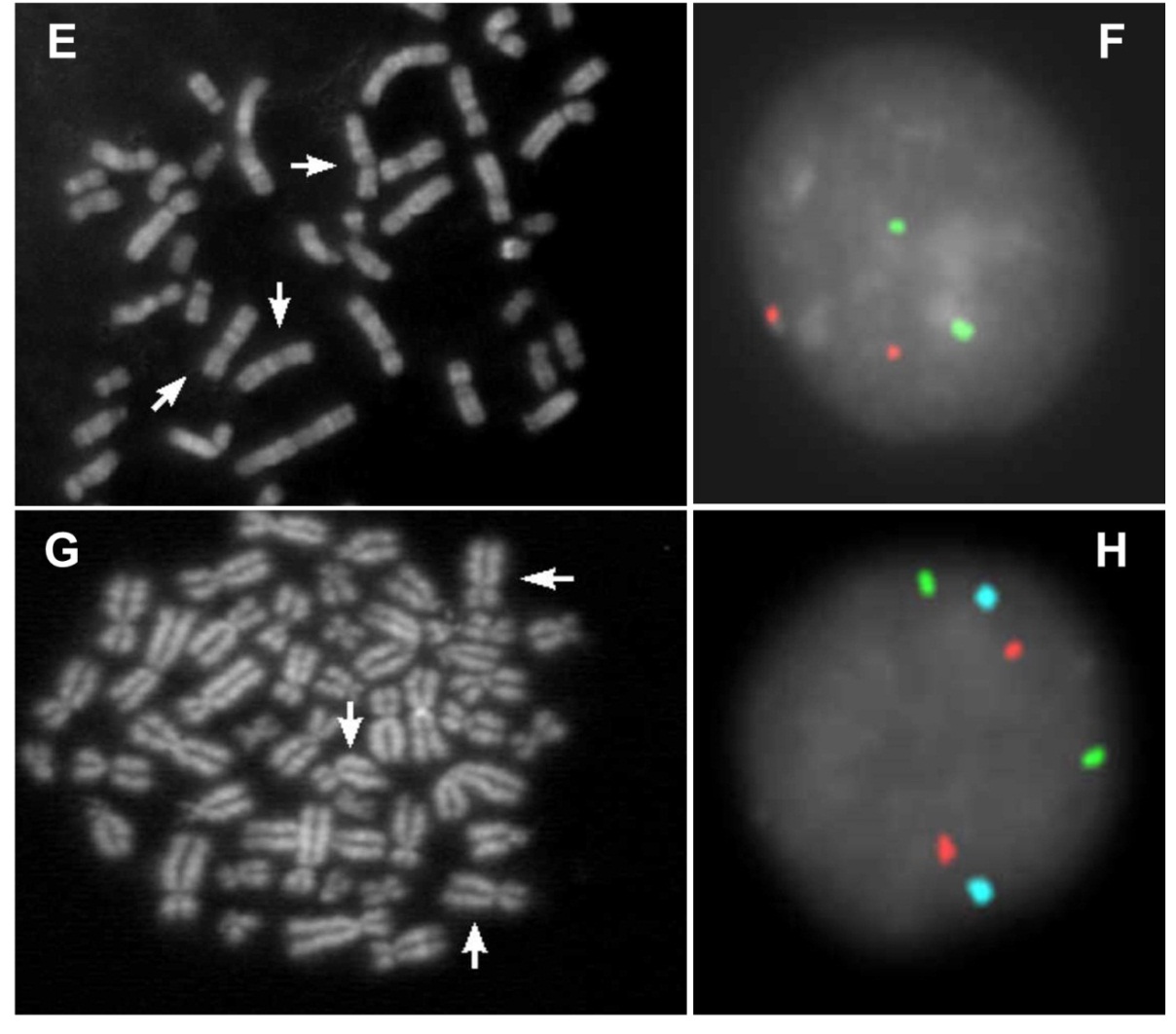
Figure 3 FISH analyses of cases 3, 4, 5, and 6. A: QFQ banded metaphase with trisomy of chromosome 13 (arrows). B: Interphase FISH analysis of amniotic fluid cells with commercial probes specific for chromosomes 13 (green) and 21 (red) (CytoCell). C: Partial QFQ banded metaphase with trisomy of chromosome 11 (arrows). D: Interphase FISH analysis of amniotic fluid nuclei with the D11Z1 commercial probe (red) (CytoCell). E: Partial QFQ banded metaphase with trisomy of chromosome 7 (arrows). F: Interphase FISH analysis of amniotic fluid nuclei with commercial probes specific for CEP7 (green) and CEP3 (red) as control (Vysis). G: QFQ banded metaphase with trisomy of chromosome 8 (arrows). H: Interphase FISH analysis of amniotic fluid cells with LSI IGH (green), LSI MYC (red), and CEP8 (aqua) probes (Vysis).
5. Interphase FISH and Contiguous Gene Syndromes
The term "contiguous gene syndrome" identifies a genetic condition characterized by small deletions or duplications, generally submicroscopic, that contain different genes and that are associated with a recognizable phenotype [18]. Some of these syndromes may have peculiar identifiable characteristics in utero through a careful fetal morphological study. If the clinical suspicion proves to be suggestive of contiguous gene syndrome, interphase FISH with a locus-specific probe allows rapid detection of a microdeletion.
Case 7
A 29-year-old woman underwent amniotic fluid sampling at the 21st week of gestation for fetal ventricular septal defect, upon the suspicion of 22q11.2 microdeletion syndrome. Due to the advanced gestational age, only interphase FISH with the TUPLE1 locus probe was performed. FISH analysis revealed disomic signals for the control probe (ARSA locus) and monosomy for the TUPLE1 region in 100 nuclei scored (Figure 4A). The 22q11.2 microdeletion was confirmed by array-CGH (Figure 4B).
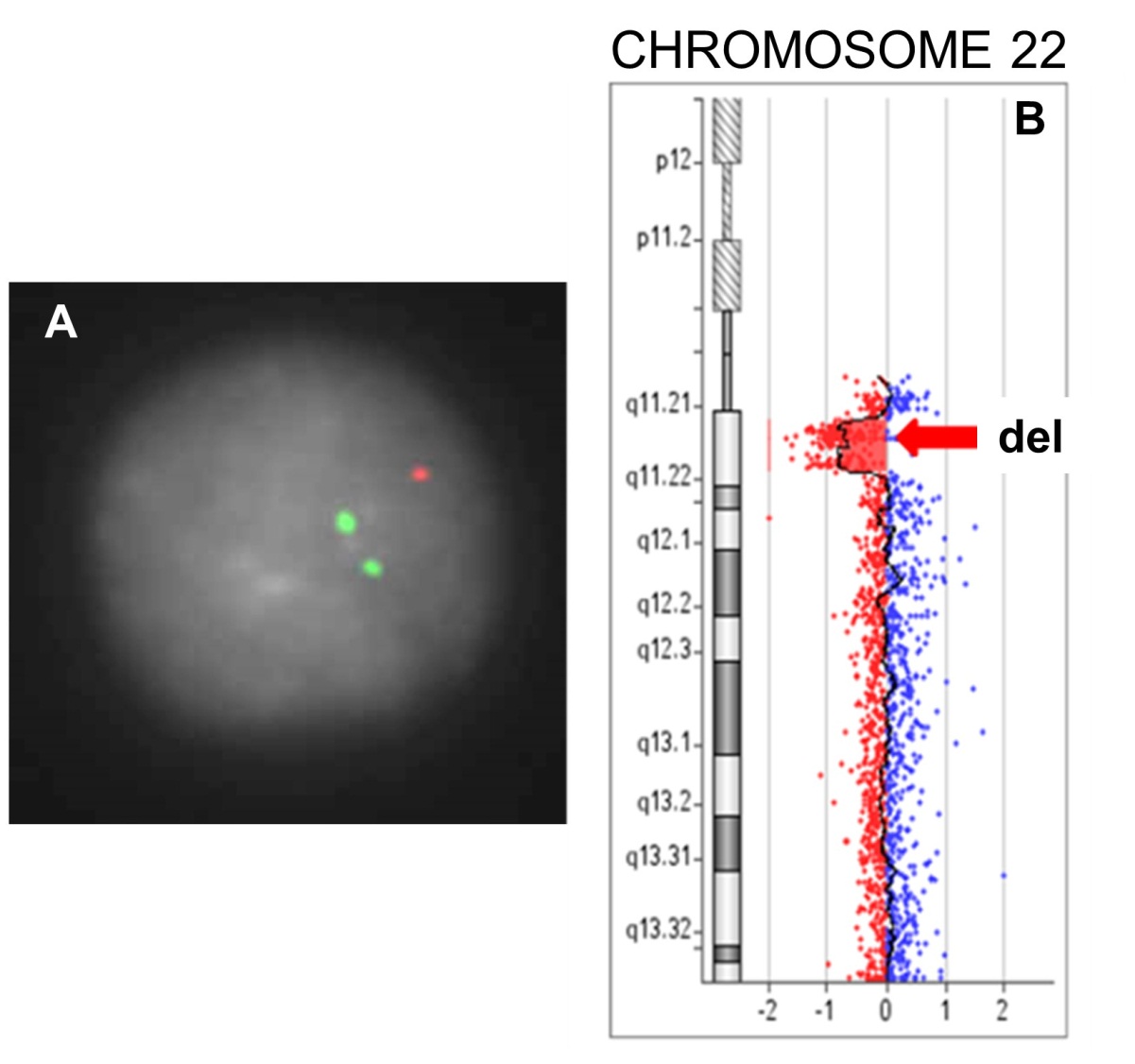
Figure 4 Case 7 deletion. A: Interphase FISH analysis of amniotic fluid nuclei with the LSI TUPLE1 (red, 22q11.2) probe and the LSI ARSA (green, 22q13.3) probe as control (Vysis). B: Deletion of the 22q11.2 chromosome region (arrow) in array-CGH analysis.
6. Conclusions
Microscope analysis of metaphases obtained after cell culture is the standard method for the study of the prenatal karyotype since its introduction in the early 1970s. The conventional karyotype is the technique of choice for the diagnosis of aneuploidy and of balanced or unbalanced structural alterations greater than 10-15 Mb, but requires about 7-10 days for its determination. Over time, various other molecular and molecular cytogenetic techniques have proven to be appropriate for overcoming and improving the three most critical aspects of the study of fetal karyotypes: the achievable resolution level, the time needed to obtain analyzable metaphases, and the number of available cells.
In the reported cases, the use of FISH has allowed the labelling of target DNA sequences and notably reduced the turnaround time (TAT) to 24-48 hours. Additionally, an important advantage of FISH is the ability to observe both metaphase and interphase nuclei. This option extends the number of feasible observations, which considers every single cell instead of mixing all cells together as in molecular techniques. Furthermore, the morphology of interphase nuclei makes it possible to distinguish between the different cell types. For example, in uncultured samples, maternal nuclei, lymphocytes, and granulocytes can be excluded, while in cultured samples, epithelial nuclei, fibroblast nuclei, and amniotic fluid nuclei of fetal origin are able to be differentiated.
FISH has proven to be a reproducible and reliable method for the evaluation of some genetic conditions that can be difficult to analyze with just the karyotype (low level mosaicism, microdeletions, and microduplication syndromes) or are not identifiable with molecular methods (cryptic translocations). Moreover, the application of FISH to non-cultured cells decreases analysis time to a few hours from the sample collection, allowing quicker treatment in the case of a pathological karyotype finding. The availability of many commercial probes from different genomic regions makes this method versatile, and its use can be modulated to address different needs that emerge during pregnancy. In our experience, this approach permits a better management of pregnancy and limits the increasing anxiety of women in the pathways of diagnosis and fetal care.
Author Contributions
All authors contributed to the review of the literature, writing and editing of the manuscript.
Funding
No funding was received for this article.
Competing Interests
The authors declare that no competing interests exist.
References
- Gall JG and Pardue ML. Molecular hybridization of radioactive DNA to the DNA of cytological preparations. Proc Natl Acad Sci USA. 1969; 63: 378-383. [CrossRef] [Google scholar] [PubMed]
- Lohn H, Birnstiel ML, Jones KW. RNA-DNA hybrids at the cytological level. Nature. 1969; 223: 582-587. [CrossRef] [Google scholar] [PubMed]
- Locatelli A, Mariani S, Ciriello E, Dalprà L, Villa N, Sala E, et al. Role of FISH on uncultured amniocytes for the diagnosis of aneuploidies in the presence of fetal anomalies. Fetal Diagn Ther. 2005; 20: 1-4. [CrossRef] [Google scholar] [PubMed]
- Lewin P, Kleinginger P, Bazin A, Mossafa H, Szipito-Tapia S. Defining the efficiency of fluorescence in situ hybridization on uncultured amniocytes on a retrospective cohort of 27407 prenatal diagnoses. Prenat Diagn. 2000; 20: 1-6. [CrossRef] [Google scholar]
- Pergament E, Chen PX, Thangavelu M, Fiddler M. The clinical application of interphase FISH in prenatal diagnosis. Prenat Diagn. 2000; 20: 215-220. [CrossRef] [Google scholar]
- Homer J, Bhatt S, Huang B, Thangavelu M. Residual risk for cytogenetic abnormalities after prenatal diagnosis by interphase fluorescence in situ hybridization (FISH). Prenat Diagn. 2003; 23: 566-571. [CrossRef] [Google scholar] [PubMed]
- Nicolini U, Lalatta F, Natacci F, Curcio C, Bui TH. The introduction of QF-PCR in prenatal diagnosis of fetal aneuploidies: time for reconsideration. Hum Reprod Update. 2004; 10: 541-548. [CrossRef] [Google scholar] [PubMed]
- Caine A, Maltby AE, Parkin CA, Waters JJ, Crolla JA; UK Association of Clinica Cytogeneticists (ACC). Prenatal detection of down's syndrome by rapid aneuploidy testing for chromosomes 13, 18, and 21 by FISH or PCR without a full karyotype: a cytogenetic risk assessment. Lancet. 2005; 366: 123-128. [CrossRef] [Google scholar] [PubMed]
- Evangelidou P, Alexandrou A, Moutafi M, Ioannides M, Antoniou P, Koumbaris I, et al. Implementation of high resolution whole genome array CGH in the prenatal clinical setting: advantages, challenges, and review of the literature. Biomed Res Int. 2013; 346762. [CrossRef] [Google scholar] [PubMed]
- Petersen AK, Cheung SW, Smith JL, Bi W, Ward PA, Peacock S, et al. Positive predictive value estimates for cell-free noninvasive prenatal screening from data of a large referral genetic diagnostic laboratory. Am J Obstet Gynecol. 2017; 217: 691.e1-691.e6. [CrossRef] [Google scholar] [PubMed]
- Costa CA. Non‐invasive prenatal screening for chromosomal abnormalities using circulating cell-free fetal DNA in maternal plasma: Current applications, limitations and prospects. Egypt J Med Hum Genet. 2017; 18: 1-7. [CrossRef] [Google scholar]
- Hsu LYF, Kaffe S, Jenkins EC, Alonso L, Benn PA, David K, et al. Proposed guidelines for diagnosis of chromosome mosaicism in amniocytes based on data derived from chromosome mosaicism and pseudomosaicism studies. Prenat Diagn. 1992; 12: 555-573. [CrossRef] [Google scholar] [PubMed]
- Hsu LYF, Benn PA. Revised guidelines for the diagnosis of mosaicism in amniocytes. Prenat Diagn. 1999; 19: 1081-1090. [CrossRef] [Google scholar]
- Kalousek DK. Pathology of abortion: chromosomal and genetic correlations. Monog Pathol. 1991; 33: 228-256. [Google scholar]
- Gardner RJM, Sutherland GR, Shaffer LG. Chromosome abnormalities and genetic conselling. 4th Ed Oxford University Press; 2012. [Google scholar]
- Hsu LYF, Yu MT, Neu RL, Van Dyke DL, Benn PA, Bradshaw CL, et al. Rare trisomy mosaicism diagnosed in amniocytes, involving an autosome other than chromosomes 13, 18, 20, and 21: karyotype/phenotype correlations. Prenat Diagn. 1997; 17: 201-242. [CrossRef] [Google scholar]
- Srinivasan A, Wright D. Pallister-killian syndrome. Am J Case Rep. 2014; 7: 194-198. [Google scholar]
- Pereira E, Marion R. Contiguous gene syndromes. Pediatr Rev. 2018; 39: 46-49. [CrossRef] [Google scholar] [PubMed]



