The Effect of the Human Plasma Molecule GHK-Cu on Stem Cell Actions and Expression of Relevant Genes
Loren Pickart *![]() , Anna Margolina
, Anna Margolina![]()
VSkin Biology, Research & Development Department, 4122 Factoria Boulevard, SE Suite No. 200 Bellevue, WA 98006, USA
* Correspondence: Loren Pickart ![]()
Academic Editors: Luis Martinez and Michael Fossel
Received: June 8, 2018 | Accepted: August 2, 2018 | Published: August 16, 2018
OBM Geriatrics 2018, Volume 2, Issue 3 doi:10.21926/obm.geriatr.1803009
Recommended citation: Pickart L, Margolina A. The Effect of the Human Plasma Molecule GHK-Cu on Stem Cell Actions and Expression of Relevant Genes. OBM Geriatrics 2018;2(3):009; doi:10.21926/obm.geriatr.1803009.
© 2018 by the authors. This is an open access article distributed under the conditions of the Creative Commons by Attribution License, which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is correctly cited.
Abstract
Background: Stem cell technology is a promising research area with a potential to create effective therapies for many degenerative diseases. However, to apply stem cell technology, we need to be able to identify and understand mechanisms that distinguish healthy regeneration processes from processes, which may result in chronic inflammation, scarring, fibrosis or cancer. GHK-Cu (glycine-L-histidine-lysine) is a small copper-binding peptide, which has a remarkable and well-documented ability to improve wound healing and tissue regeneration, regulate remodeling of connective tissue and synthesis of collagen, elastin and glycosaminoglycans, reduce inflammation and scarring, increase antioxidant-enzymes and protect cells from toxic by-products of lipid peroxidation.
Methods: Authors used a computer-based gene profiling tool, The Connectivity Map, to identify a number of human genes regulated by GHK, relevant to regulation of cell differentiation, apoptosis and stem cell function.
Results: The number of human genes associated with stem cell function was 57 genes in the range of increases of 50% UP and 46 genes in the range of decreases of 50% DOWN.
Conclusion: Based on laboratory data and gene profiling data, GHK-Cu may be used to improve stem cell therapy and to help shift regeneration processes to healthy regeneration.
Keywords
GHK-Cu; stem cells; matrikines; wound healing; tissue regeneration; gene profiling
1. Introduction
With senior populations growing in many countries, stem cells attract more and more attention as a promising resource in anti-aging therapies. Their ability to repair tissues, which plays a key role in restoring an organ’s functionality after an injury, puts them in the forefront of current research in regenerative medicine.
There are three main types of stem cells – embryonic stem cells, adult stem cells and cancer stem cell-like cells. Embryonic stem cells are true omnipotent stem cells, which makes them ideal candidates for regenerative medicine treatments; however, their use is hindered by ethical considerations. Therefore, understanding adult stem cells and harnessing their regenerative potential have become important goals in current regenerative medicine research [1].
A promising source of adult stem cells for stem cell therapy is from epidermal stem cells residing in skin. As recent studies indicate, epidermal stem cells’ behavior depends on their niche. When skin is intact, resident epidermal stem cells are committed to their differentiation programs. However, when skin is injured, stem cells are able to migrate from their niche and change their differentiation program, acquiring a high degree of plasticity [2].
Another branch of current research is focused on stem cell-like cells found in certain types of cancer, which are believed to be the cells actively involved in metastasis, tumor invasion and cancer relapse. The mechanisms by which cancer growth can induce stemness in senescent cells are currently investigated [3].
Up until recently, it was believed that cellular senescence, which slows down and stops cell division may be a protective mechanism against cancer. However, a surprising discovery revealed that cellular senescence may elicit stemness, and that this mechanism plays an important role in cancer relapse [4,5].
It is increasingly apparent that tissue regeneration is a process that shares many mechanisms with cancer growth. In order to restore the tissue’s integrity, not only do resident stem cells have to be activated, but also some differentiated cells must revert to a stem cell-like state and acquire mobility. In inflammation and cancer growth, certain inflammatory mediators such as IL 6 and IL 8, can induce stemness in senescent cells [6]. Some pathways that are activated during the wound healing process and inflammation can lead to an increase in cancer stem cell-like population. So, molecules that reduce inflammation and improve regulation of the cytokines and pathways in the course of the wound healing process can help prevent cancer growth [7].
There is increasing understanding of the complexity of how genes regulate functions of stem cells [8]. Regeneration often is accompanied by inflammation and reorganization of the tissue architecture. In order to do this, certain genes that previously were silent, or down-expressed, have to be activated, while others have to be silenced. Today, researchers have an unprecedented opportunity to access gene profiling databases and determine which genes are affected during various physiological and pathological processes. These databases, such as The Broad Institute Connectivity Map, allow researchers to analyze gene expression changes caused by various biologically active molecules [9].
Also, it is now discovered that inflammation and the accompanying generation of free radicals of oxygen (ROS) can trigger tissue plasticity by reverting cells to pluripotency [10]. Studies show that inflammation in the prostate may expand the pool of progenitor-like stem cells that are prone to malignant transformation [11].
Therefore, one of the primary focuses of regenerative medicine research is identifying and understanding mechanisms that distinguish healthy regeneration processes, which lead to restoration of normal tissue architecture, and pathological processes, which may result in chronic inflammation, scarring, fibrosis or cancer. One of the mechanisms that are currently investigated is the Wnt signalling pathway. A normally functioning Wnt signalling pathway ensures tissue regeneration and maintenance of tissue integrity, while deregulated Wnt plays a role in aging and cancer [12]. Another key player is the p53 gene, which is now recognized as a cancer suppressor and important regulator of microenvironment in the stem cell niche [13].
It is established that stem cells are influenced by their niche and take cues from their microenvironment. A stem cell niche is a microenvironment in the extracellular matrix in which a stem cell resides. The extracellular matrix components affect growth, differentiation and mobility of stem cells through a multitude of peptide regulators, adhesions molecules, growth factors and transcriptional factors [14]. Among these regulators, small regulatory peptides, often described under an umbrella term “matrikines,” attract particular interest [15].
Matrikines (or matrycryptins) are small peptides that are released from a tissue after an injury, during an enzymatic cleavage of tissue proteins. Matrikines are attractive candidates for future anti-cancer and tissue regeneration-enhancing drugs; however, the application is hindered by the complexity of their interaction [16]. One of the potentially promising approaches, in our opinion, is identifying molecules that have “the master-key” qualities. One of the most promising candidates is the copper-binding peptide GHK (glycine-L-histidine-lysine) [17]. This peptide discovered in 1973 by Pickart, has the highest concentration in young adults (age 25 and younger), when tissues have the most regenerative potential; however, it declines with age [18].
Early studies by Maquart et al. established that GHK stimulates wound healing and tissue regeneration, increases collagen, elastin and glycosaminoglycans. Since then, multiple animal and in vitro studies confirmed GHK-Cu’s ability to improve wound healing and tissue regeneration [19,20]. The positive effects of GHK-Cu were confirmed for many organs and systems such as skin, lungs, liver, intestinal lining, nervous system and bones. The effects of GHK cover a wide range of physiological processes, from regeneration and wound healing to anxiolytic, anti-aggression and analgesic effects [21,22,23,24,25]. GHK increases the level of antioxidant enzymes and has an anti-inflammatory effect [26]. The effects of GHK-Cu are summarized in Table 1.
The real breakthrough happened in 2010, when Hong at al. used the Broad Institute gene response database The Connectivity Map to identify the top molecules capable of reversing gene expression characteristic for metastatic colon cancer. Two molecules, GHK and securinine topped the list and were selected out of 1,309 bioactive molecules as the best agents capable to reverse expression of 54 gene sets overexpressed in malignant invasive metastatic colon cancer, which included node molecules YWHAB, MAP3K5, LMNA, APP, GNAQ, F3, NFATC2, and TGM2, involved in regulation of multiple biochemical pathways [27]. Surprisingly, both molecules happened to also be stimulators of tissue regeneration. This study brought attention to GHK’s ability to modulate expression of multiple genes relevant to cancer suppression and tissue regeneration.
Table 1 Biological effects of GHK-Cu

In 2012, Campbell et al. demonstrated that GHK can reverse gene expression characteristic for COPD (Chronic Obstructive Lung Disease). This study is of particular interest because the researchers also paired gene studies with laboratory studies, confirming that changes in gene expression are associated with improved collagen reorganization and fibroblasts function [28] Further studies confirmed the ability of GHK-Cu to reverse gene expression in COPD back to health [29]. These studies provided an intriguing link between GHK’s role in cancer prevention, tissue regeneration and stem cells.
The current paper reviews GHK’s effect on gene expression relevant to stem cell function and regeneration.
2. Materials and Methods
The authors used the Broad Institute's Connectivity Map (CMap) - a publicly available computer-based gene profiling tool. The Connectivity Map is a database that contains more than 7,000 gene expression profiles of 5 human cell lines treated with 1,309 distinct small molecules [30].
The GHK profiles, contained in this repository, are based on PC3 human prostate cancer cells and MCF7 human breast cancer cells. In order to analyze the gene data obtained from the CMap, we used GenePattern - a publicly available computational biology open-source software package developed for the analysis of genomic data. The CEL files were processed with MAS5 and background correction. Files were then uploaded to the ComparativeMarkerSelectionViewer module in order to view fold changes for each probe set. The Gene Ontology descriptions of the molecular function, biological process, and cellular component of gene products was used to obtain GHK induced gene expression results relevant to stem cells.
Due to multiple probe sets mapping to the same gene, we converted the fold changes in m-RNA production produced by GenePattern to percentages, then averaged all probe sets representing the same gene. It was determined that the 22,277 probe sets in the Broad data represent 13,424 genes. This ratio (1.66) was used to calculate the overall number of genes that affect GHK at various cutoff points (rather than probe sets). This method has been described in our previous publications, where we presented our data on genes relevant to nervous system function, antioxidant system, ubiquitin-proteosome system, skin health and tissue regeneration affected by GHK [31,32,33,34]. This paper presents gene profiling data relevant to GHK’s effects on stem cells function.
It is important to notice that The Broad Institute gene profiling was performed with GHK, not GHK-Cu. However, considering GHK’s high affinity for copper (see below) and essentiality of copper for cell growth, it is virtually impossible to have copper-free GHK in a living system. If ample copper 2+ is available in a test system or an animal, GHK should easily obtain necessary copper.
3. Results
The number of human genes associated with stem cell function was 57 genes in the range of increases of 50% UP and 46 genes in the range of decreases of 50% DOWN. Table 2 summarizes GHK’s effects on genes relevant to cell cycle regulation. Table 3 presents genes that are upregulated, and Table 4 presents genes that are down regulated.
Table 2 Genes relevant to cell cycle regulation upregulated by GHK

Table 3 Genes relevant to stem cell function upregulated by GHK

Table 4 Gene relevant to stem cells function suppressed by GHK

4. Discussion
4.1 GHK and Gene Expression Relevant to Stem Cell Function
An examination of the GHK-induced actions (Tables 2-4) on gene expression relevant to stem cell function finds many genes that control development and differentiation, cell growth, RNA and DNA synthesis and transcription. For some genes, the Broad Institute database gives more than one value and they are averaged in the tables.
In some cases, it appears that GHK induces a selection pattern among genes with similar functions. For example, GHK suppresses gene ACBC1 (ATP-binding Cassette) by 1,536% but only suppresses a similar gene ACCB4 by only 70%. Likewise, gene ING2, which functions in DNA repair and apoptosis, is increased by 336% while gene PIWIL2, which eliminates germ lines with DNA damage is suppressed by 214 %. Also, there is the problem that the function of many genes is still poorly defined.
It is known that GHK is a normal constituent of human plasma and may be a breakdown product of collagen. In addition, it is generated from SPARC (secreted protein acidic and rich in cysteine). The expression of SPARC is minimal in most normal adult tissues but is increased after injury. Proteolysis of this protein by plasmin generates GHK [35].
We propose that during wound repair, the initially generated GHK is copper free and stimulates stem cell replication. Later, as GHK accumulates bound copper 2+, GHK-Cu stimulates stem cell differentiation.
4.2 GHK and Stem Cells
The first suggestion that GHK might induce stem cell production arose from wound healing studies. Burn surgeons have long observed that the influx of hair follicles into a burned area predicts a good healing response. It is now established that dermal hair follicles provide a major source of stem cells used for dermal healing. Stem cells for the skin are thought to arise from enlarged hair follicles. The first indication that GHK affects stem cells came from mouse studies where GHK-Cu produced a very strong amplification of hair follicle size (see the description below). A similar peptide, Ala-His-Lys-copper 2+, produced even stronger actions [36].
The mouse in Figure 1 was shaved, then treated in three spots with GHK-Cu. The result is a much more rapid hair growth (the three circular patches of hair) in the spots treated with copper peptides. In the microscopic images, the magnifications are identical. The top photo is the untreated mouse skin – the control. The bottom photo is mouse skin treated with GHK-Cu. Note the larger hair follicles (elongated columns) in the lower photo, the increased content of subcutaneous fat in the skin (white material in the center of the skin), and the increased thickness of the skin. Hair researchers have noted the accumulation of such fat around healthy follicles that are vigorously growing hair and its relative lack around dormant follicles. They have postulated that these cells serve a supportive function for the hair follicle. It must be emphasized that effects in humans on hair follicle health are not as dramatic.
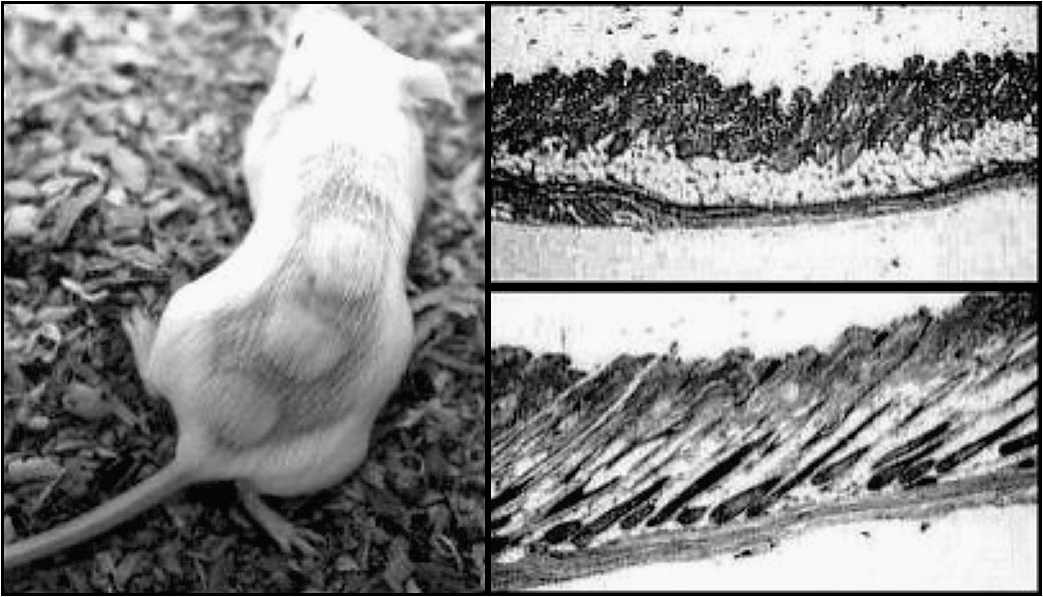
Figure1 GHK-Cu was tested for effects of hair growth in 30-day old Swiss-Webster mice. Control mice were treated with 0.85% saline in water. On the left: A mouse was shaved and treated with GHK-Cu on three areas. On the right top: Control - 0.85% saline-treated skin. On the right bottom: GHK-Cu treated skin.
4.3 GHK-Cu’s Possible Mechanism of Action
Despite decades of research, there is still a lack of unanimous agreement among researchers as to what allows GHK to produce so many diverse and positive biological effects. In 1980, Pickart proposed that GHK acts by regulating copper intake into the cell [37]. Copper is an essential element in the human body, and it is involved in multiple biochemical processes which affect cell growth and differentiation, tissue regeneration, nervous system health and development, and gene expression [38]. Since then, biological effects of GHK have been commonly attributed to its role in copper metabolism.
There are approximately 700 albumin molecules in human plasma for each GHK molecule, so GHK does not play a significant role in copper 2+ transport. However, GHK has some unique characteristics which may be attributed to its importance as a copper-regulating compound on a cellular level. It has been established that GHK has a very high affinity for copper 2+ and can easily obtain the ionic metal from its transport site on human albumin. A comparison of copper binding properties, copper exchange rate and redox potential between two similar naturally occurring copper-binding peptides GHK and DAHK shows that even though both bind copper in stoichiometry at a ratio of 1:1, the exchange rate of copper between DAHK peptides is very slow, while the exchange rate between GHK peptides is much faster. Both complexes are inert under moderate redox conditions and do not increase oxidation [39]. Due to its small size and unique copper-binding characteristics, GHK may be able to facilitate rapid exchange of copper in the intracellular space.
Even though, considering the essentiality of copper in humans, the copper hypothesis of GHK mechanism of action seems very attractive, recent gene data may challenge this mechanism and demand more studies into GHK’s mechanism of action.
4.4 GHK, Copper and Stem Cells
Stem cell proliferation requires extremely low copper concentrations that are created by the use of copper chelating agents. However, when stem cells are exposed to higher copper levels, they progress into differentiated cells. Peled and colleagues, members of a stem cell biotech firm (Gamida Cell, Jerusalem, Israel), has claimed, in a patent, that GHK increases proliferation of stem cells, while GHK-Cu increases their progression into differentiated cells [40]. In 2005, Peled and colleagues claimed that copper-free GHK maintained the clonogenic potential of stem cells while GHK-Cu increased cell copper by 2,162 % above the control value and caused stem cell differentiation [41].
Further supporting the above are the findings that low tissue copper, in itself, may increase stem cell proliferation and availability in animals. For instance, copper deprivation contributes to neogenesis of alpha and beta cells in the pancreatic ducts of diabetic rats [42].
Feeding diabetes-prone BioBreeding (BBdp) rats a hydrolyzed casein-based diet, a diet that binds nutritional copper and lowers tissue copper, promotes islet cell neogenesis and results in 2–3-fold fewer diabetes cases compared with feeding cereal-based diets [43].
Jose et al. found that the human plasma copper binding peptide GHK added to cultures of mesenchymal stem cells increased their concentrations of VEGF and basic fibroblast growth factor, and also increased endothelial cell proliferation, migration and tubule formation. GHK also had no apparent cytotoxic effects on MSC in culture over a wide range of concentrations [44].
4.5 Anti-inflammatory Activity of GHK-Cu
As stated above, inflammation is one of the factors that can disrupt wound healing and trigger abnormal cell plasticity. There is an ample evidence that GHK-Cu possesses a strong anti-inflammatory effect.
GHK administered intraperitoneally at doses of 2.6, 26, and 260 μg/ml/day reduced inflammatory cell infiltration, levels of TNF-α and IL-6, as well as abolished bleomycin-induced elevation of TGF-beta in bleomycin-induced lung fibrosis in mice. The authors concluded that GHK acts by affecting TGF-β1/Smad 2/3 and IGF-1 pathway. [45].
GHK protected lungs from lipopolysaccharide-induced damage by suppressing infiltration of inflammatory cells into lungs and reducing inflammatory response. It also suppressed NF-κB p65 and p38 MAPK signaling pathways and protected the lungs by reducing reactive oxygen species (ROS) production, stimulating superoxide dismutase (SOD), while decreasing TNF-α and IL-6 production [46].
Also, GHK and GHK-Cu decreased TNF-alpha-dependent secretion of pro-inflammatory IL-6 in normal human dermal fibroblasts NHDF cell line [47].
4.6 DNA Repair
In our previous publications [29], we identified genes relevant to DNA repair that were positively affected by GHK (Table 5).
Table 5 Genes relevant to DNA repair affected by GHK
|
Gene title (GENE database) |
Comments |
Percent change in gene expression |
|
PARP3, Poly (ADP-ribose) polymerase family, member 3 |
PARP3 enzymes play role in DNA repair, regulation of apoptosis, and maintenance of genomic stability |
+253 |
|
POLM, Polymerase (DNA directed), mu |
DNA repair enzyme |
+225 |
|
RAD50, RAD50 double strand break repair protein |
DNA repair enzyme, double strand break repair |
+175 |
|
RARA, Retinoic acid receptor, alpha |
Regulates transcription |
+123 |
4.7 GHK vs GHK-Cu
There are many studies that obtain significant results with copper-free GHK, but the copper chelator bathocuproine abolishes the GHK effects. It is difficult to remove trace amounts of copper ions from the amino acids mixtures used in cell culture media.
Two studies from Seoul National University (Republic of Korea) found the effects of GHK-Cu and GHK copper-free on cell differentiation on a skin equivalent cell culture system produced identical results. Both molecules (0.1–10 μM) stimulated the proliferation of keratinocytes in a dose dependent manner and resulted in changes in epidermal basal cells whose integrins and p63 expression markedly increased. Epidermal cells became more cuboidal, as is characteristic for stem cells. The authors concluded that GHK and GHK-Cu increased epidermal cell stemness and keratinocyte proliferation, also increasing p63 and integrin expression [48,49].
In most of our animal studies, we used a mixture of 2 molecules of GHK to 1 molecule of copper ion to avoid the possible oxidative actions of free copper ion.
5. Conclusions
Recently, many compounds, discovered through computer-based gene-profiling, were proposed as novel therapeutics. In our opinion, when considering a new compound for clinical testing, it is important to have in vivo and in vitro studies confirming positive effects suggested by gene profiling as well as safety. GHK-Cu is a compound which has a long history of safe usage in skin care and which has been studied extensively in animal experiments, and in cell and tissue culture studies. Recent gene profiling studies allow deeper understanding of mechanisms of its positive actions.
Complex influences in stem cells’ microenvironment determine whether the stem cell will function normally, improving tissue repair and regeneration, or transform into a malignant cell. Stem cell senescence also depends on gene expression and microenvironmental cues. Both laboratory and gene studies suggest that GHK, a very safe molecule and low-cost molecule, could improve stem cell therapies.
The Lethal Dose of GHK-Cu for 50% of mice (LD50) deaths was 8 mg for a 25-gram mouse or about 23 grams for a 70 kg human. A possible explanation of lethal effects of high doses of GHK-Cu is dropping of blood pressure. There are no reports for an LD50 for GHK without copper, so it must be very nontoxic. However, penicillamine, a copper chelator used in Wilson's Disease, has been reported to, at times, both induce psychosis or reverse psychosis.
For experimental human use, dissolved GHK could be added to the stem cell mixture. A total of 10 mg in an adult human should be adequate, which is about 2000 times lower than the LD50 calculated for humans. Recent Russian studies of rats observed a reduction of anxiety and pain induced aggression 12 minutes after intraperitoneal administration of GHK at concentrations that would correspond to 35 micrograms in a 70 kg human.
To improve skin regeneration GHK can be used in creams, gels and skin patches, since it can penetrate the stratum corneum [50]. It can be delivered directly into the affected site through the use of microneedles [51]. Also, GHK can be easily incorporated in liposomes and used as a dietary supplement [52].
Acknowledgments
Authors want to thank Germaine Pugh and Cassia McClain for their invaluable help in preparing the manuscript, and Jessica Michelle Vasquez-Soltero for processing the gene profiling data.
Author Contributions
Authors equally contributed to research and preparation of the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
References
- Blanpain C, Fuchs E. Stem Cell Plasticity. Plasticity of epithelial stem cells in tissue regeneration. science. 2014; 13: 1242281. doi: 10.1126/science.1242281 [CrossRef] [Google scholar] [PubMed]
- Gonzales KAU, Fuchs E. Skin and its regenerative powers: an alliance between stem cells and their niche. Dev Cell. 2017; 20: 387–401. [CrossRef] [Google scholar] [PubMed]
- Milanovic M, Fan DNY, Belenki D, Däbritz JHM, Zhao Z, Yu Y, et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018; 553: 96-100. [CrossRef] [Google scholar] [PubMed]
- Dou Z, Berger SL. Senescence elicits stemness: a surprising mechanism for cancer relapse. Cell Metab. 2018; 27: 710-711. [CrossRef] [Google scholar] [PubMed]
- Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017; 15: 172-183. [CrossRef] [Google scholar] [PubMed]
- Ortiz-Montero P, Londoño-Vallejo A, Vernot JP. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun Signal. 2017; 15: 17. doi: 10.1186/s12964-017-0172-3. [CrossRef] [Google scholar] [PubMed]
- Arnold KM, Opdenaker LM, Flynn D, Sims-Mourtada J. Wound healing and cancer stem cells: inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis. 2015; 8: 1-13. [CrossRef] [Google scholar] [PubMed]
- Yang Z, Balic A, Michon F, Juuri E, Thesleff I. Mesenchymal wnt/β‐Catenin signaling controls epithelial stem cell homeostasis in teeth by inhibiting the antiapoptotic effect of fgf10. Stem Cells. 2015; 33: 1670-1681. [CrossRef] [Google scholar] [PubMed]
- Brum AM, Van de Peppel J, Nguyen L, Aliev A, Schreuders-Koedam M, Gajadien T, et al. Using the connectivity map to discover compounds influencing human osteoblast differentiation. J Cell Physiol. 2018; 233: 4895-4906. [CrossRef] [Google scholar] [PubMed]
- Meng S, Chanda P, Thandavarayan RA, Cooke JP. Transflammation: how innate immune activation and free radicals drive nuclear reprogramming. Antioxid Redox Signal. 2018; 29: 205-218. [CrossRef] [Google scholar] [PubMed]
- Liu X, Grogan TR, Hieronymus H, Hashimoto T, Mottahedeh J, Cheng D, et al. Identifies progenitor-like inflammation-associated luminal cells that can initiate human prostate cancer and predict poor outcome. Cell Rep. 2016; 17: 2596-2606. [CrossRef] [Google scholar] [PubMed]
- Perochon J, Carroll LR, Cordero JB. Wnt signalling in intestinal stem cells: lessons from mice and flies. Genes (Basel). 2018; 9: 138. [CrossRef] [Google scholar] [PubMed]
- Olivos DJ, Mayo LD. Emerging non-canonical functions and regulation by p53: p53 and stemness. Int J Mol Sci. 2016; 17: 1982. [CrossRef] [Google scholar] [PubMed]
- Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a mynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014; 1840: 2506-2519. [CrossRef] [Google scholar] [PubMed]
- Gaur M, Dobke M, Lunyak VV. Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int J Mol Sci. 2017; 18. doi:10.3390/ijms18010208. [CrossRef] [Google scholar] [PubMed]
- Ricard-Blum S, Vallet SD. Matricryptins network with matricellular receptors at the surface of endothelial and tumor cells. Front Pharmacol. 2016; 7. doi: 10.3389/fphar.2016.00011. [CrossRef] [Google scholar] [PubMed]
- Maquart FX, Siméon A, Pasco S, Monboisse C. Regulation of cell activity by the extracellular matrix: the concept of matrikines. J Soc Biol. 1999; 193: 423-428. [CrossRef] [Google scholar]
- Pickart L. A Tripeptide from human serum which enhances the growth of neoplastic hepatocytes and the survival of normal hepatocytes. San Francisco: University of California; 1973. [Google scholar]
- Pickart L, Margolina A. Anti-aging activity of the GHK peptide—the skin and beyond. J Aging Res Clin Pract. 2012; 1: 13–16. [Google scholar]
- Badenhorst T, Svirskis D, Merrilees M, Bolke L, Wu, Z. Effects of GHK-Cu on MMP and TIMP expression, collagen and elastin production, and facial wrinkle parameters. J Aging Sci. 2016; 4. doi: 10.4172/2329-8847.1000166. [CrossRef] [Google scholar]
- Bobyntsev II, Chernysheva OI, Dolgintsev ME, Smakhtin MY, Belykh AE. Anxiolytic effects of Gly-His-Lys peptide and its analogs. Bull Exp Bio Med. 2015; 158: 726-728. [CrossRef] [Google scholar] [PubMed]
- Sever’yanova LА, Dolgintsev ME. Effects of tripeptide Gly-His-Lys in pain-induced aggressive-defensive behavior in rats. Bull Exp Bio Med. 2017; 164: 140-143. [CrossRef] [Google scholar] [PubMed]
- Bobyntsev II, Chernysheva OI, Dolgintsev ME, Smakhtin M, Belykh AE. Effect of Gly-His-Lys peptide and its analogs on pain sensitivity in mice. Eksp Klin Farmakol. 2015; 78: 13-15. [Google scholar]
- Chernysheva OI, Bobyntsev II, Dolgintsev ME. The tripeptide GLY-HIS-LYS influence on behavior of rats in the test “open field”. Biological Sci. 2014; 12: 357-360. [Google scholar]
- Pickart L, Vasquez-Soltero JM, Margolina A. Resetting skin genome back to health naturally with GHK. Textbook of Aging Skin, 2014: 1549-1566. [Google scholar]
- Pickart L, Vasquez-Soltero JM, Margolina A. GHK-Cu may prevent oxidative stress in skin by regulating copper and modifying expression of numerous antioxidant genes. Cosmetics, 2015; 2: 236-247. [CrossRef] [Google scholar]
- Hong Y, Downey T, Eu KW, Koh PK, Cheah PY. A ‘metastasis-prone’signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clinical & Exp Metastasis. 2010; 27: 83-90. [CrossRef] [Google scholar] [PubMed]
- Campbell JD, McDonough JE, Zeskind JE, Hackett TL, Pechkovsky DV, Brandsma CA, et al. A gene expression signature of emphysema-related lung destruction and its reversal by the tripeptide GHK. Genome Med. 2012; 4: 67. doi: 10.1186/gm367. [CrossRef] [Google scholar] [PubMed]
- McMurry R, Perdomo C, Liu G, Zhang S, Stevenson C, Campbell J, et al. GHK-Cu elicits in vitro, dose-dependent transcriptional alterations in pathways relevant to extracellular matrix composition. Am J Resp Critical Care Medicine, 2017; 195: A2446. [Google scholar]
- Lamb J. The connectivity map: a new tool for biomedical research. Nat Rev Cancer. 2007; 7: 54-60. [CrossRef] [Google scholar] [PubMed]
- Pickart L, Vasquez-Soltero JM, Margolina A. The effect of the human peptide GHK on gene expression relevant to nervous system function and cognitive decline. Brain Sci. 2017; 7. doi: 10.3390/brainsci7020020. [CrossRef] [Google scholar] [PubMed]
- Pickart L, Vasquez-Soltero JM, Margolina A. GHK and DNA: resetting the human genome to health. BioMed Res Int. 2014; 151479. doi: 10.1155/2014/151479. [CrossRef] [Google scholar] [PubMed]
- Pickart L, Margolina A. Regenerative and protective actions of the GHK-Cu peptide in the light of the new gene data. Int J Mol Sci. 2018; 19. doi: 10.3390/ijms19071987. [CrossRef] [Google scholar] [PubMed]
- Pickart L, Vasquez-Soltero JM, Margolina A. GHK peptide as a natural modulator of multiple cellular pathways in skin regeneration. BioMed Res Int. 2015; 648108. doi: 10.1155/2015/648108 [CrossRef] [Google scholar] [PubMed]
- Sage EH, Vernon RB. Regulation of angiogenesis by extracellular matrix: the growth and the glue. J Hypertens Suppl. 1994; 12: 145-152. [Google scholar]
- Pickart L. The human tri-peptide GHK and tissue remodeling. J Biomaterials Sci. 2008; 19: 969-988. [CrossRef] [Google scholar] [PubMed]
- Pickart L, Freedman JH, Loker WJ, Peisach J, Perkins CM, Stenkamp RE, et al. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature. 1980; 288: 715-717. [CrossRef] [Google scholar] [PubMed]
- Uauy R, Olivares M, Gonzalez M. Essentiality of copper in humans. Am J Clin Nutr. 1998; 67: 952-959. [CrossRef] [Google scholar] [PubMed]
- Hureau C, Eury H, Guillot R, Bijani C, Sayen S, Solari PL, et al. X-ray and solution structures of Cu(II) GHK and Cu(II) DAHK complexes: influence on their redox properties. Chemistry. 2011; 17: 10151-10160. [CrossRef] [Google scholar] [PubMed]
- Peled T, Landau E, Prus E, Treves AJ, Fibach E. Cellular copper content modulates differentiation and self‐renewal in cultures of cord blood‐derived CD34+ Cells. Br J Haematol. 2002; 116: 655-661 [CrossRef] [Google scholar] [PubMed]
- Peled T, Fibach E, Treves A. U.S. Patent No. 7,855,075. Washington: Patent and Trademark Office. 2010. [Google scholar]
- Al-Abdullah IH, Ayala G, Panigrahi D, Kumar A M, Kumar MSA. Neogenesis of pancreatic endocrine cells in copper-deprived rat models. Pancreas. 2000; 21: 63-68. [CrossRef] [Google scholar] [PubMed]
- Wang GS, Gruber H, Smyth P, Pulido O, Rosenberg L, Duguid W,et al. Hydrolysed casein diet protects BB rats from developing diabetes by promoting islet neogenesis. J Autoimmun. 2000; 15: 407-416. [CrossRef] [Google scholar] [PubMed]
- Jose S, Hughbanks ML, Binder BY, Ingavle GC, Leach JK. Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomater. 2014; 10: 1955-1964. [CrossRef] [Google scholar] [PubMed]
- Zhou XM, Wang GL, Wang XB, Liu L, Zhang Q, Yin Y, et al. GHK peptide inhibits bleomycin-Induced pulmonary fibrosis in mice by suppressing TGFβ1/Smad-mediated epithelial-to-mesenchymal transition. Front Pharmacol. 2017; 904. doi: 10.3389/fphar.2017.00904. [CrossRef] [Google scholar] [PubMed]
- Park JR, Lee H, Kim SI, Yang SR. The tri-peptide GHK-Cu complex ameliorates lipopolysaccharide-induced acute lung injury in mice. Oncotarget. 2016; 7: 58405-58417. [CrossRef] [Google scholar] [PubMed]
- Gruchlik A, Jurzak M, Chodurek E, Dzierzewicz Z. Effect of Gly-Gly-His, Gly-His-Lys and their copper complexes on TNF-alpha-dependent IL-6 Secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012; 69: 1303-1306. [Google scholar]
- Kang YA, Choi HR, Na JI, Huh CH, Kim MJ, Youn SW, et al. Copper-GHK increases integrin expression and p63 positivity by keratinocytes. Arch Dermatol Res. 2009; 301: 301–306. [CrossRef] [Google scholar] [PubMed]
- Choi HR, Kang YA, Ryoo SJ, Shin JW, Na JI, Huh CH, et al. Stem cell recovering effect of copper-free GHK in skin. J Pept Sci. 2012; 18: 685-690. [CrossRef] [Google scholar] [PubMed]
- Hostynek JJ, Dreher F, Maibach HI. Human skin retention and penetration of a copper tripeptide in vitro as function of skin lyer towards anti-inflammatory therapy. Inflamm Res 2010, 59: 983–988. [CrossRef] [Google scholar] [PubMed]
- Li H, Low YS, Chong HP, Zin MT, Lee CY, Li B, et al. Microneedle-Mediated delivery of copper peptide through skin. Pharm Res. 2015; 32: 2678-2689 [CrossRef] [Google scholar] [PubMed]
- Erdem S, Türkoglu M. Glycyl-L-Histidyl-L-Liysine-Cu(2+) loaded liposome formulations. Marmara Pharm J. 2010; 14: 91–97. [CrossRef] [Google scholar]


